Figures & data
Figure 1. Primary congenital glaucoma (PCG). (a) Two patients with PCG show buphthalmos and varying degrees of corneal edema (white arrows). Patient 2 also has a backwards ‘C’ shaped haabs striae (black arrows). (b) Optic nerve photographs of a patient with PCG at presentation (top row) and 4 years after obtaining IOP control from angle surgery (bottom row) show reversal of cupping (white arrowheads) especially in the left eye.
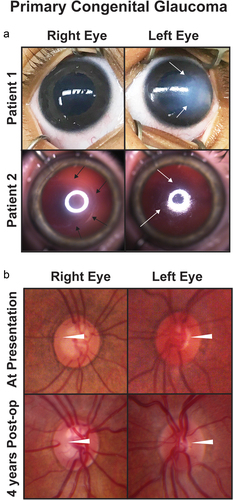
Figure 2. Aniridia. Slit lamp photos (top row) and backlit photos (bottom row) of a patient with aniridia shows keratopathy (white arrows) which is worse in the right eye the the left eye. In the right eye, the white arrows point to the same areas of keratopathy in both the slit lamp and backlit photographs. In the left eye, the white arrows points to a peripheral pannus that is more obvious in the backlit photograph. Both eyes have a superotemporal glaucoma drainage device (asterisk) which due to the proxmity to the lens has caused a focal cataract (black arrow).
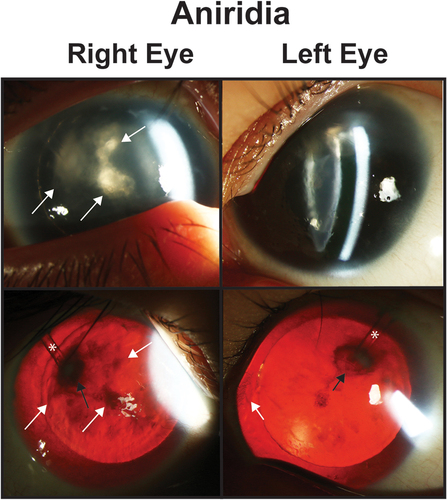
Figure 3. Axenfeld-Rieger Syndrome (ARS) and Iridogoniodysgenesis. Patients 1 and 2 have classic with posterior embryotoxon (white arrows) and iris hypoplasia leading to corectopia and pseudopolycoria. Both patients have superotemporal and inferornasal glaucoma drainage devices (asterisks). Patient 3 has prominent posterior embryotoxon (white arrows) and ectropion uvea with iris stromal atrophy (white arrowhead). Patient 4 has iridogoniodysgenesis associated with a FOXC1 mutation. The iris is hypoplasitic (black arrow) and a trabeculectomy with mitomycin C (black arrowhead) had previously been performed for glaucoma.
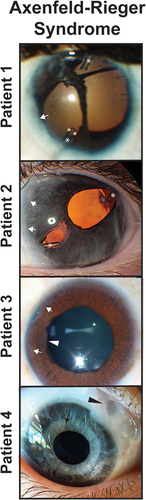
Figure 4. Congenital corneal opacities. (a) Bilateral peters anomaly with a denser opacity in the right eye than the left eye. Ultrasound biomicroscopy demonstrates iridocorenal adhesions in the area of the corneal defect (white arrows). (b) Sclerocornea with microphthalmia showing a small hazy cornea with unclear demarcation of the limbus. The left eye has a superior iris opening which underlies an area of clearer cornea.
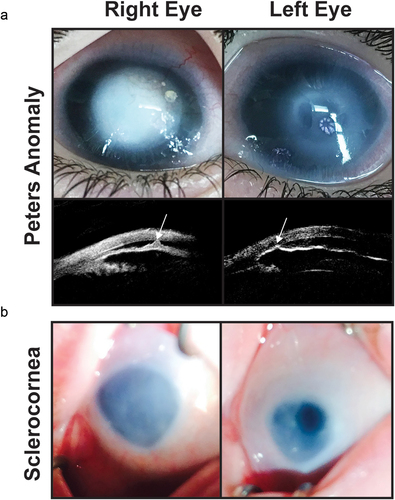
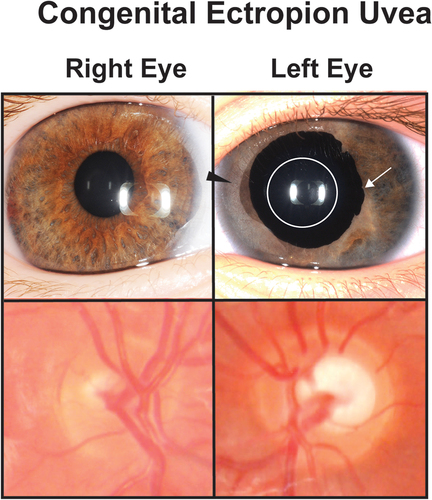
Figure 5. Congenital ectropion uvea (CEU). A patient with unilateral congenital ectropion uvea (left eye) shows the iris epithelium hypertrophy (white arrow) around the pupil (white circle). The iris also shows stromal atrophy (black arrowhead) compared to the unaffected right eye. The left optic nerve is also more cupped than the right eye demonstrating the effect of increased IOP.