Figures & data
Figure 1. Global sales of intravenous iron products (iron dextran and ferric carboxymaltose) from 2008 to 2017 in million DDDs (IQVIA™ MIDAS® data).
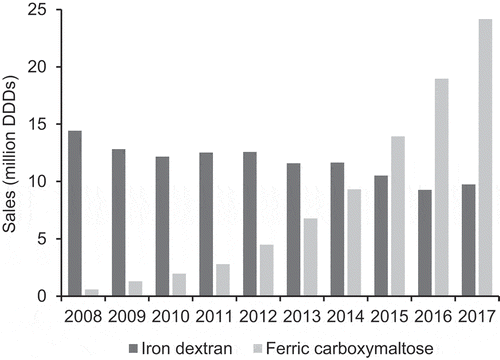
Table 1. Global reported number and rate of hypersensitivity reactions and death with iron dextran (ID) and ferric carboxymaltose (FCM).
Figure 2. Global reporting rate of hypersensitivity reactions (SMQ Anaphylactic reaction Group A–D) per 100,000 DDDs of iron dextran and ferric carboxy-maltose (VigiBase™ and IQVIA™ MIDAS® data).
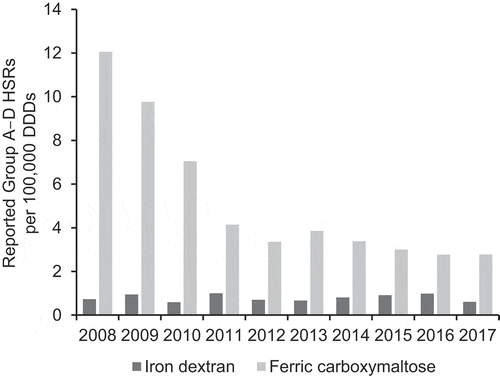
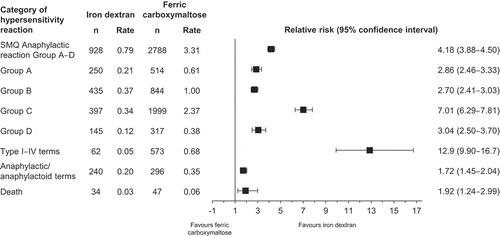
Figure 3. Global reported number, rate, and relative risk of hypersensitivity reactions and death with iron dextran and ferric carboxymaltose from 2008 to 2017. Abbreviations: DDD, defined daily dose; Group A, narrow terms pertaining to hypersensitivity reactions; Group B, broad terms pertaining to respiratory reactions potentially related to hypersensitivity; MedDRA®, Medical Dictionary for Regulatory Activities; n, number of reported adverse drug reactions (VigiBase™ data); rate, number of reported adverse drug reactions per 100,000 DDDs; SMQ, Standardized MedDRA® Query.