Figures & data
Table 1. Advantages and disadvantages of current and potential therapies for NTDT
Figure 1. Molecular pathways of iron-restricted erythropoiesis through translational control at eIF2#x3B1;P, TfR2/Scrib and mTORC1 signaling. Reproduced from Zhang S, et al. Blood 2018;131(4):450–461 [39], with permission from the American Society of Hematology. #xA9; 2018 by the American Society of Hematology. Left: Steady-state erythropoiesis in the bone marrow under iron sufficiency. In iron and thus heme abundance, HRI homodimer is inactive because of full occupancies of heme onto the 4 HRI heme-binding domains and so is unable to phosphorylate eIF2#x3B1;, thus permitting global protein synthesis, mainly globin proteins in erythroid cells. Sufficient hemoglobin production maintains oxygen-delivering capacity in blood without hypoxia. Middle: Activation of HRI-ISR under iron deficiency mitigates ineffective erythropoiesis. Under iron/heme deficiency, HRI in bone marrow erythroid precursors is activated by the dissociation of heme. HRI then induces ISR, phosphorylating eIF2#x3B1;, which inhibits globin protein synthesis and results in a decrease of hemoglobin content and consequently induction of tissue hypoxia stress. In addition, eIF2#x3B1;P selectively enhances the translation of ATF4 mRNA to alleviate ROS levels. HRI-ISR also inhibits mTORC1 signaling to mitigate ineffective erythropoiesis in the spleen. Right: Elevated mTORC1 signaling and development of ineffective erythropoiesis in mutant mice defective in HRI-ISR signaling in iron deficiency. Hypoxia induced by iron deficiency stimulates Epo production in the kidney and increases Epo in blood circulation. In the spleen, binding of Epo to its receptors in erythroid precursors induces AKT/mTORC1 signaling, thus phosphorylating 4EBP1 and S6K/S6 to increase protein synthesis, promote proliferation, and inhibit erythroid differentiation, which are the characteristics of ineffective erythropoiesis. HRI-ISR serves as feedback to inhibit mTORC1 signaling activity, inhibiting the development of ineffective erythropoiesis in iron deficiency. 4EBP1, eukaryotic translation initiation factor 4E binding protein 1; AKT, protein kinase B; ATF4, activating transcription factor 4; eIF2#x3B1;, eukaryotic translation initiation factor 2 subunit 1; Epo(R), erythropoietin (receptor); Fe, iron; HRI-ISR, heme-regulated eIF2#x3B1; kinase-integrated stress response; mRNA, messenger ribonucleic acid; mTORC1, mammalian target of rapamycin complex 1; P, phosphorylated; ROS, reactive oxygen species; Scrib, scribble; S6(K), ribosomal protein S6 (kinase); TfR2, transferrin receptor 2.
![Figure 1. Molecular pathways of iron-restricted erythropoiesis through translational control at eIF2#x3B1;P, TfR2/Scrib and mTORC1 signaling. Reproduced from Zhang S, et al. Blood 2018;131(4):450–461 [39], with permission from the American Society of Hematology. #xA9; 2018 by the American Society of Hematology. Left: Steady-state erythropoiesis in the bone marrow under iron sufficiency. In iron and thus heme abundance, HRI homodimer is inactive because of full occupancies of heme onto the 4 HRI heme-binding domains and so is unable to phosphorylate eIF2#x3B1;, thus permitting global protein synthesis, mainly globin proteins in erythroid cells. Sufficient hemoglobin production maintains oxygen-delivering capacity in blood without hypoxia. Middle: Activation of HRI-ISR under iron deficiency mitigates ineffective erythropoiesis. Under iron/heme deficiency, HRI in bone marrow erythroid precursors is activated by the dissociation of heme. HRI then induces ISR, phosphorylating eIF2#x3B1;, which inhibits globin protein synthesis and results in a decrease of hemoglobin content and consequently induction of tissue hypoxia stress. In addition, eIF2#x3B1;P selectively enhances the translation of ATF4 mRNA to alleviate ROS levels. HRI-ISR also inhibits mTORC1 signaling to mitigate ineffective erythropoiesis in the spleen. Right: Elevated mTORC1 signaling and development of ineffective erythropoiesis in mutant mice defective in HRI-ISR signaling in iron deficiency. Hypoxia induced by iron deficiency stimulates Epo production in the kidney and increases Epo in blood circulation. In the spleen, binding of Epo to its receptors in erythroid precursors induces AKT/mTORC1 signaling, thus phosphorylating 4EBP1 and S6K/S6 to increase protein synthesis, promote proliferation, and inhibit erythroid differentiation, which are the characteristics of ineffective erythropoiesis. HRI-ISR serves as feedback to inhibit mTORC1 signaling activity, inhibiting the development of ineffective erythropoiesis in iron deficiency. 4EBP1, eukaryotic translation initiation factor 4E binding protein 1; AKT, protein kinase B; ATF4, activating transcription factor 4; eIF2#x3B1;, eukaryotic translation initiation factor 2 subunit 1; Epo(R), erythropoietin (receptor); Fe, iron; HRI-ISR, heme-regulated eIF2#x3B1; kinase-integrated stress response; mRNA, messenger ribonucleic acid; mTORC1, mammalian target of rapamycin complex 1; P, phosphorylated; ROS, reactive oxygen species; Scrib, scribble; S6(K), ribosomal protein S6 (kinase); TfR2, transferrin receptor 2.](/cms/asset/cb30cd26-a37d-44bf-850f-d0f561943343/ierr_a_1935854_f0001_oc.jpg)
Table 2. Summary of drugs in clinical development targeting the hepcidin–ferroportin axis
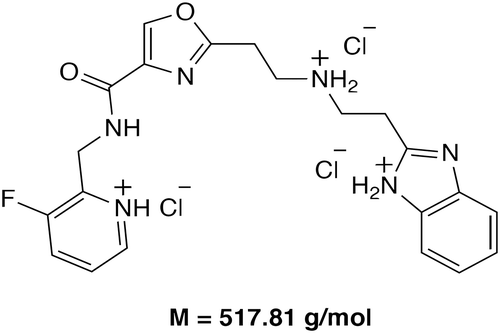
Table 3. Key findings from preclinical studies of vamifeport (VIT-2763)
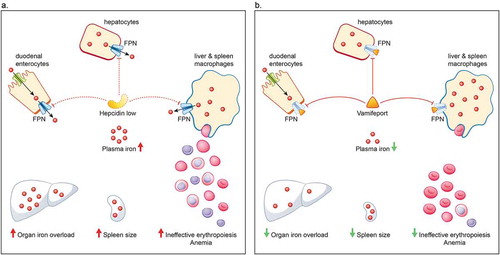
Figure 3. Hepcidin–ferroportin axis in non-transfusion-dependent thalassemia (A) and how it is affected by vamifeport (VIT-2763) (B). A. Iron homeostasis is mediated primarily via the hepcidin–ferroportin axis. Hepcidin regulates iron levels by targeting the iron transporter ferroportin for degradation. Erythropoiesis exerts a strong control over iron homeostasis, so that when erythropoiesis is impaired, iron absorption is increased, irrespective of iron stores. In patients with NTDT, anemia and hypoxia arising from ineffective erythropoiesis stimulates erythropoiesis and suppresses hepcidin. The reduced level of hepcidin causes an increase in the absorption of dietary iron and release of recycled iron from macrophages, ultimately resulting in saturation of the iron-binding capacity of transferrin and consequently generation of non-transferrin-bound iron that results in organ iron overload and further drives ineffective erythropoiesis, splenomegaly, and anemia. B. Vamifeport, a novel oral ferroportin inhibitor, acts like hepcidin to target ferroportin for degradation, thereby blocking iron export into plasma. By limiting plasma iron levels, vamifeport acts to prevent organ iron overloading, reduce spleen size, and improve erythropoiesis and anemia. FPN, ferroportin; NTDT, non-transfusion-dependent thalassemia