Figures & data
Figure 1. Mechanism of action of brentuximab vedotin. Reproduced with permission from Suri Citation12. © 2018 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc., on behalf of American Society for Clinical Pharmacology and Therapeutics.
Abbreviation: MMAE, monomethyl auristatin E
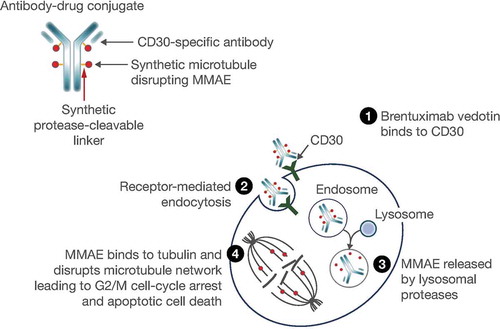
Table 1. Demographics and baseline disease characteristics (safety/mITT population)
Table 2. Summary of best overall response (mITT population)
Figure 2. Swimmer plot of response to treatment from date of first dose by disease type (mITT population). (a) cHL and (b) sALCL.
Abbreviations: BOR, best overall response; CR, complete response; EOT, end of treatment; cHL, classical Hodgkin lymphoma; LTFU, long-term follow-up; mITT, modified intent to treat; PD, progressive disease; PR, partial response; sALCL, systemic anaplastic large cell lymphoma; SCT, stem cell transplant; SD, stable disease; sub anti-trt, subsequent antineoplastic therapy
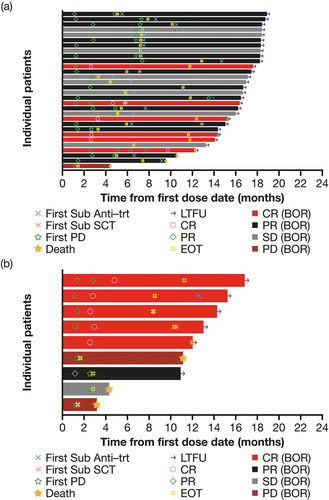
Figure 3. Best percentage change in the sum of product diameters by disease type (mITT population; all patients). (a) cHL and (b) sALCL.
Abbreviations: CR, complete response; cHL, classical Hodgkin lymphoma; mITT, modified intent to treat; PD, progressive disease; PR, partial response; sALCL, systemic anaplastic large cell lymphoma; SD, stable disease; SPD, sum of product diameters
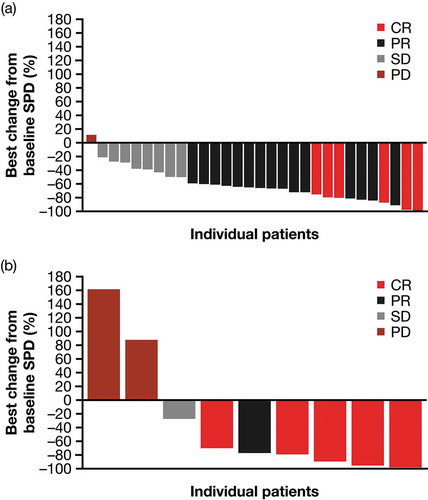
Figure 4. Kaplan–Meier curves for patients with cHL (left panels) and sALCL (right panels). Illustrating (a) and (b) duration of response (mITT population with CR or PR). (c) and (d) progression-free survival (mITT population). (e) and (f) overall survival (mITT population).
Abbreviations: CI, confidence interval; cHL, classical Hodgkin lymphoma; CR, complete response; mITT, modified intent to treat; NE, not estimable; ORR, overall response rate; sALCL, systemic anaplastic large cell lymphoma
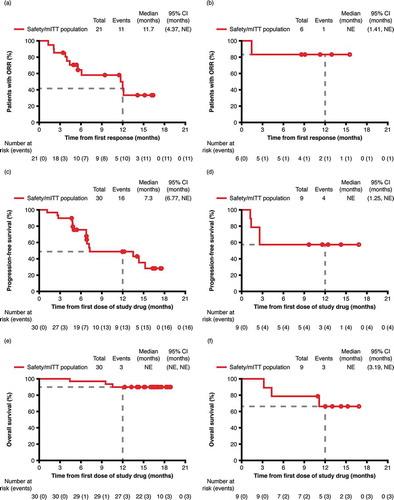
Table 3. Summary of AEs (safety population)
Table 4. Summary of resolution and improvement of PN at EOT (safety population)
Supplemental Material
Download MS Word (141.9 KB)Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article, will be made available within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
