Figures & data
Table 1. Estimated prevalence, correlation of factor level with clinical presentation, and availability of a single clotting factor concentrate of selected ultra-rare inherited bleeding disorders.
Table 2. Members of Working Group 3, by subgroup.
Figure 1. Working Group 3 research priorities for ultra-rare inherited bleeding disorders schematic of community-identified areas for priority research framework POC: point of care.
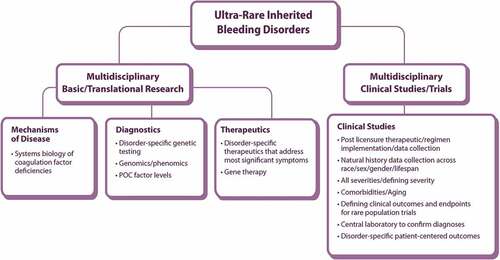
Figure 2. Questions and elements/modalities considered by the WG3 subgroup to address community priorities in diagnostics, systems biology, and mechanistic science of ultra-rare inherited bleeding disorders.

Figure 3. Questions and elements/modalities considered by WG3 to address clinical, data collection, and research infrastructure community priorities for ultra-rare inherited bleeding disorders.

Figure 4. Questions and elements/modalities considered by WG3 to address community priorities in regulatory processes for novel therapeutics and required data collection for ultra-rare inherited bleeding disorders.

Figure 5. Plot of feasibility, impact, and risk scores of the questions evaluated by the three WG3 subgroups.
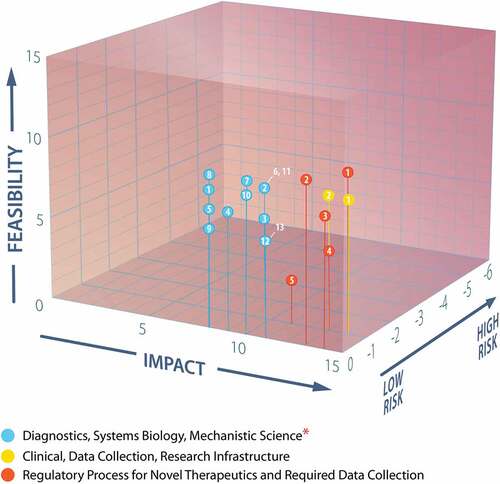
Table 3. Actionable initiatives scored for feasibility, impact, and risk in the three domains of ultra-rare inherited bleeding disorders community priorities.
