Figures & data
Figure 1. Patient disposition. Of the 18 patients who did not meet the eligibility criteria, 1 was rescreened and entered with a different patient ID. Therefore, this patient was counted twice. FVIII, factor VIII; ITI, immune tolerance induction.
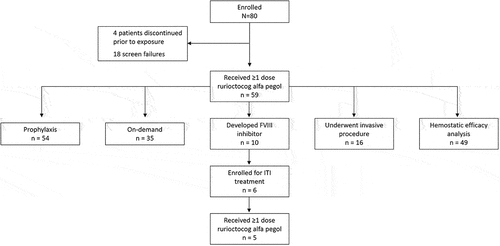
Table 1. Patient demographics and baseline characteristics.
Table 2. Patients receiving rurioctocog alfa pegol with at least 1 positive post-baseline binding IgG and IgM antibody to FVIII, PEG-FVIII, and PEG.
Table 3. Hemostatic efficacy of rurioctocog alfa pegol in prophylactic treatment and control of bleeds.
Table 4. Weight-adjusted consumption and number of infusions for patients receiving rurioctocog alfa pegol prophylaxis.
Table 5. Adverse events occurring during or after first dose of rurioctocog alfa pegol in the safety analysis set and patients who were subsequently enrolled to receive ITI.
Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results of the study, will be made available after the publication of study results within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization.
