Figures & data
Table 1. Arguments for the key role of microbes in IBD.
Figure 1. The different levels of mucosal defense against invasion of the microbiota. This expert review will focus on level I.
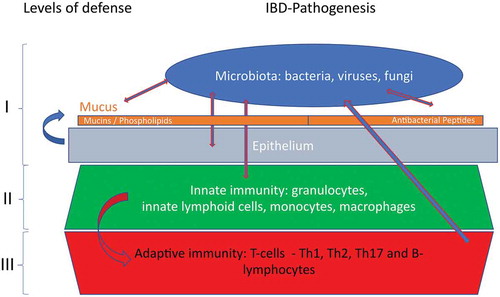
Figure 2. Possible interactions of the gut microbiota with the mucosal barrier, ultimately leading to IBD. In the left scenario, the microbiota is altered, leading to barrier defects and inflammation in a susceptible host. In the right scenario, primary mucosal barrier defects lead to altered gut microbiota, and eventually an onset of inflammation.
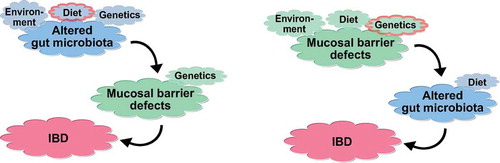
Table 2. Arguments for microbes being hen or egg in IBD.
Figure 3. Small-intestinal barrier function during homeostasis and IBD. During homeostasis, the gut microbiota is held at a distance through a mucus layer that is loaded with antimicrobial peptides (AMPs), which are predominantly produced by Paneth cells. During IBD, decreased diversity of the gut microbiota is consistently observed over most studies. Moreover, reduced antimicrobial activity and adherent-invasive E. coli (AIEC) are observed in ileal biopsies, which may be a result of defective Paneth cell function in Crohn’s disease.
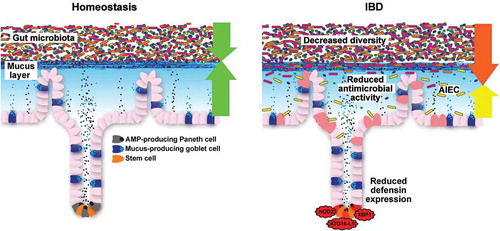
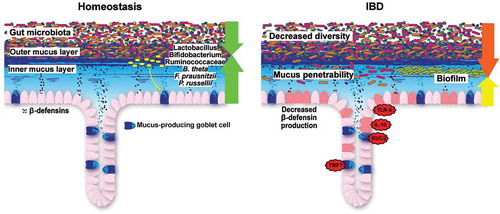
Figure 4. Colonic barrier function during homeostasis and IBD. During homeostasis, gut microbiota colonization is restricted to the outer mucus layer. Beneficial commensal bacteria, such as Lactobacillus, Bifidobacterium, Ruminococcaceae, Bacteroides thetaiotaomicron (B. theta), Faecalibacterium prausnitzii (F. prausnitzii) and Peptostreptococcus russellii (P. russellii) stimulate and maintain active mucosal barrier function. During IBD, bacterial diversity is decreased, the inner colonic mucus layer is penetrable to bacteria (ulcerative colitis) and a biofilm, mainly consisting of Bacteroides fragilis, can be observed at the mucosa (Crohn’s disease). In mice, several genetic defects recapitulate the clinical signs of ulcerative colitis.