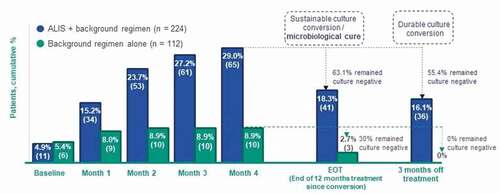
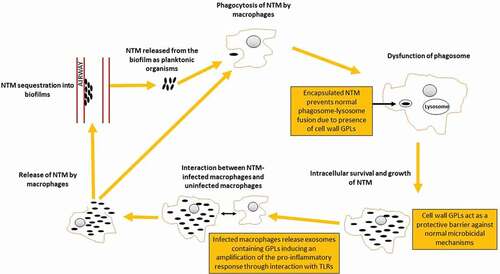
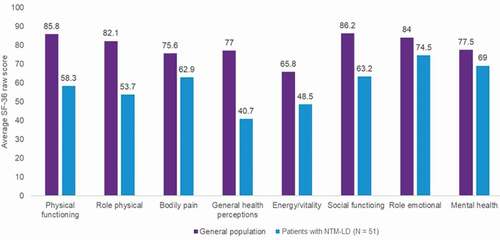
Figures & data
Table 1. Mortality Estimates Among Patients with NTM-LD Compared with Matched Controls
Table 2. Key Outcomes for Management of NTM-LD, per the NTM-NET Consensus
Table 3. 2020 ATS/ERS/ESCMID/IDSA Recommended Treatment Regimensa for MAC-LD According to Disease Status and/or Severity
![Figure 3. Depiction of Amikacin Liposome Inhalation Suspension (ALIS) Liposome Structure Source: Amikacin liposomal inhalation suspension [package insert] 2020 [Citation97]](/cms/asset/d9aa0891-af16-4f68-a58a-70f3bcaf2857/ierx_a_1987891_f0003_oc.jpg)