Figures & data
Figure 1. Molecular mechanisms underlying the therapeutic effects of mometasone furoate in asthma. Mometasone furoate is a liposoluble molecule which crosses the cell membrane and binds to the cytoplasmic glucocorticoid receptor (GR). Following mometasone binding, the activated GR migrates to the nucleus, where interacts with specific regulatory DNA sequences known as glucocorticoid response elements (GRE). GRE are located inside target genes encoding anti-inflammatory proteins such as GILZ (glucocorticoid-induced leucine zipper) and MKP-1 (mitogen-activated protein kinase phosphatase-1). The latter dephosphorylates and inactivates the pro-inflammatory p38 MAPK enzyme. Furthermore, activated GR can also bind via protein–protein interactions to the transcription factor nuclear factor-kB (NF-kB), thereby inactivating its proinflammatory functions by preventing DNA-binding.
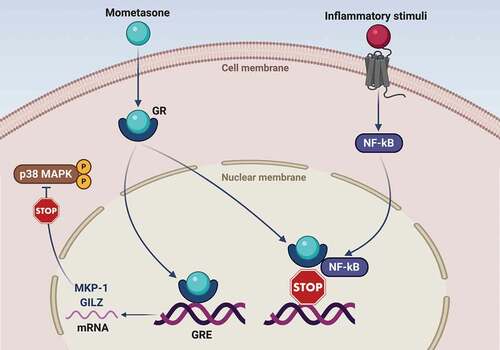
Figure 2. Molecular interactions involving the mechanisms of action of indacaterol, glycopyrronium and mometasone furoate. Indacaterol binds to the β2-adrenergic receptor (β2-AR), thus sequentially activating the stimulatory Gs protein and adenylyl cyclase (AC), which is responsible for cAMP synthesis and the subsequent activation of cAMP-dependent protein kinase A (PKA). This signaling pathway not only leads to bronchodilatation, but also promotes GR nuclear translocation, thus potentiating the pharmacologic effects of corticosteroids. The latter in turn increase β2-AR mRNA levels via GRE-dependent stimulation of β2-AR gene transcription. IND-induced bronchodilatation is synergistically potentiated by GLY through a competitive antagonism of M2 and M3 muscarinic receptors. With respect to the blockade of the M2 receptor coupled to AC inhibition operated by the inhibitory G protein (Gi), GLY occupies for a much longer time the M3 receptor, whose stimulation is the main mechanism underlying acetylcholine-induced bronchoconstriction, mediated by M3-coupling to Gq protein and phospholipase C (PLC). The latter enzyme catalyzes the generation of the intracellular second messenger inositol trisphosphate (IP3) that elicits airway smooth muscle contraction via mobilization of cytosolic calcium ions.
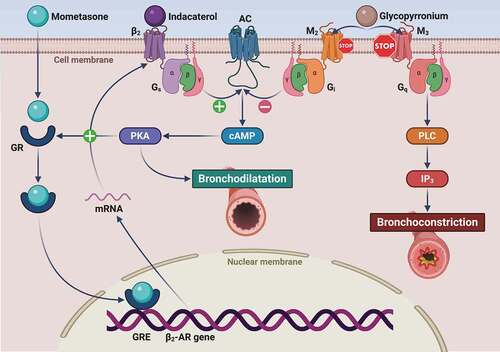
Table 1. Summary of the phase III studies within PLATINUM program
