Figures & data
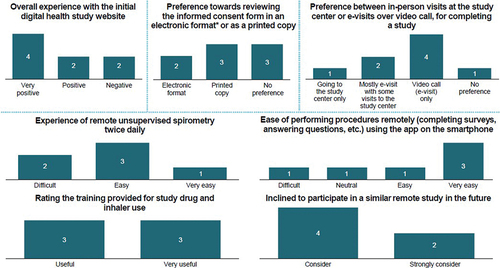
Figure 1. Mean number of exacerbations, time to first exacerbation and time to change in treatment in GOLD-compliant versus non-GOLD-compliant treatment regimens. (a) Mean number of any exacerbations per patient year; *P < 0.05; **P < 0.001; ***P < 0.0001 (negative binomial regression). (b) Mean time to first exacerbation among patients with ≥1 exacerbation; *P < 0.05; **P < 0.001; ***P < 0.0001 (t-test). (c) Mean time to first index therapy change; **P < 0.001; ***P < 0.0001 (t-test). (d) Mean time to discontinuation of index therapy; **P < 0.001; ***P < 0.0001 (t-test). GOLD, Global Initiative for Chronic Obstructive Lung Disease.
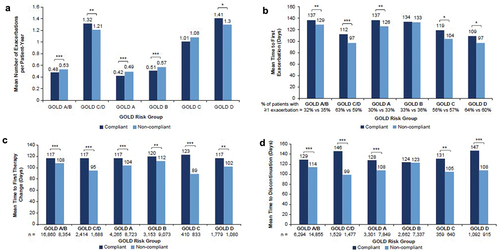
Figure 2. (a) Risk of first COPD exacerbation. (b) Risk of first hospitalization for community-acquired pneumonia.
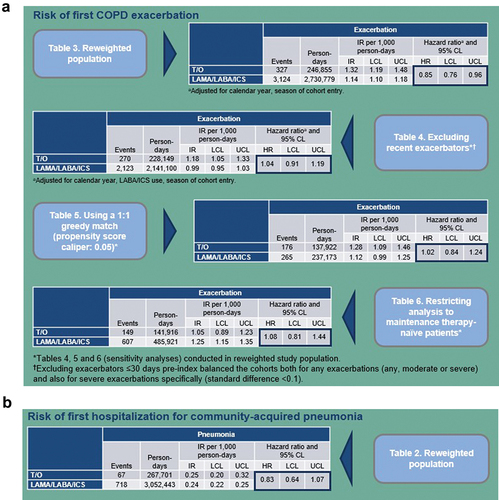
Figure 3. Kaplan-Meier analysis of time to COPD-related hospitalization among (a) treatment naïve MAPD patients and (b) MAPD patients with no exacerbation history. Measured from index date + 1, or discharge date + 1, if patient had inpatient visit crossing index date, until discontinuation of the index therapy. Diagnosis of interest must have occurred within an inpatient visit that began in the on-treatment period.
Figure 3. COPD, chronic obstructive pulmonary disease; MAPD, Medicare with Part D; PSM, propensity score matching; TIO+OLO, tiotropium/olodaterol; TT, triple therapy. Wald chi-square test using robust standard errors in a proportional hazard model was used for assessing equality of hazard rates.
Figure 4. Primary endpoint results.
Figure 4. COPD, chronic obstructive pulmonary disease; ET80%, elapsed time over which a flow of >80% of peak flow was achieved; FAS, full analysis set; IR, inspiratory resistance; L, liters; PIF, peak inspiratory flow; s, seconds; SE, standard error; Tin, total inhalation time; TPIF, time to PIF; VCin, total inhaled volume; VPIF, inhaled volume at PIF as a percentage of VCin.
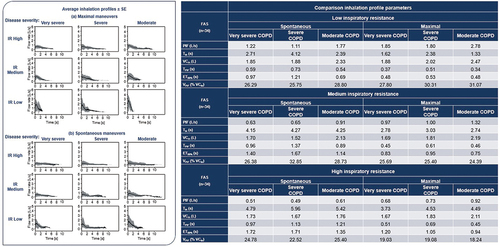
Table 1. Summary of findings