Figures & data
Figure 1. Coding tree used for analysis.
Diagram illustrating the coding tree used for qualitative content analysis. NMES device development is centrally located and connects to four different categories: stages, barriers and facilitators, and applicability to acquired dysarthria. Each category has several branches with the sub-categories associated with each.
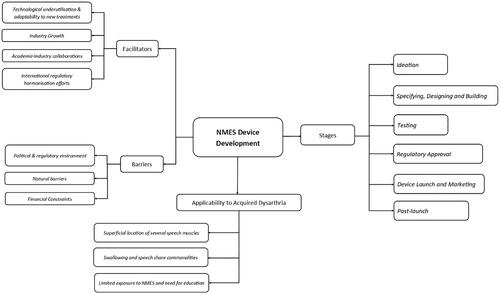
Figure 2. Simplistic schematic representation of the NMES device development process.
Schematic diagram illustrating the stages of NMES device development. Ideation is linked one-way to the specifying, designing, and building. The process then transitions to testing and regulatory approval respectively. Bidirectional arrows link the specifying, designing and building stage, the testing and the regulatory approval stages. These stages then connect and flow to device launch and marketing via a one-way arrow, which in turn is connected to post-launch. Post-launch reconnects to ideation, initiating iterative cycles.
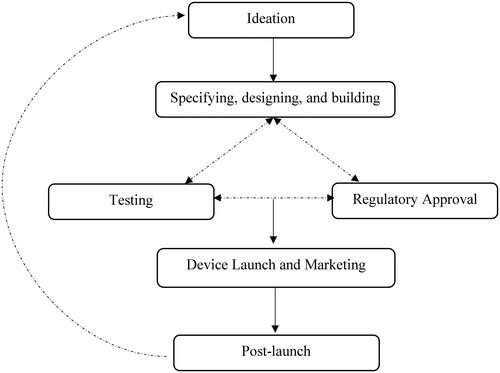
Table 1. Characteristics of participants.
Supplemental material
