Figures & data
Figure 1. The trial flow chart illustrates the novel dose-adjustment protocol and the conventional one for subcutaneous immunotherapy in allergic rhinitis due to delayed injection. Baseline: baseline before treatment; M0: the maintaining pretreatment baseline of the conventional group; R0: the maintaining pretreatment baseline of the novel dose-adjustment protocol group; M3: the end of 3-year treatment in the conventional group; R3: the end of 3-year treatment in the novel dose-adjustment protocol group.
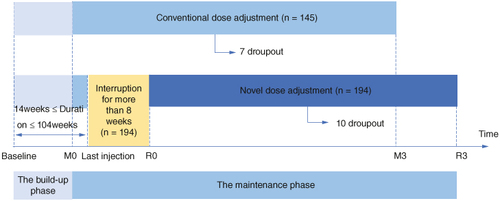
Table 1. Demographic characteristics comparison between the novel group and the conventional group.
Figure 2. Comparison of therapeutic effects between the novel dose-adjustment protocol group and the conventional protocol group in subcutaneous immunotherapy. (A) Nasal congestion score; (B) itchy nose score; (C) sneezing score; (D) runny nose score; (E) TNSS; (F) MS; (G) CSMS. Baseline: baseline before treatment; M0: the maintaining pretreatment baseline of the conventional group; R0: the maintaining pretreatment baseline of the novel dose-adjustment protocol group; M3: the end of 3-year treatment in the conventional group; R3: the end of 3-year treatment in the novel dose-adjustment protocol group.
CSMS: Combined symptom and medication score; MS: Medication score; TNSS: Total nasal symptom score.
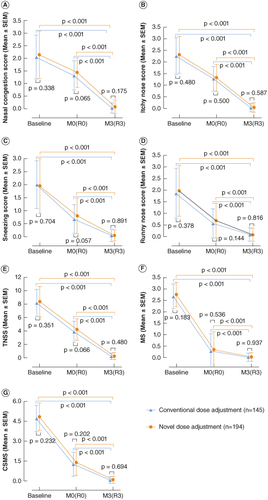
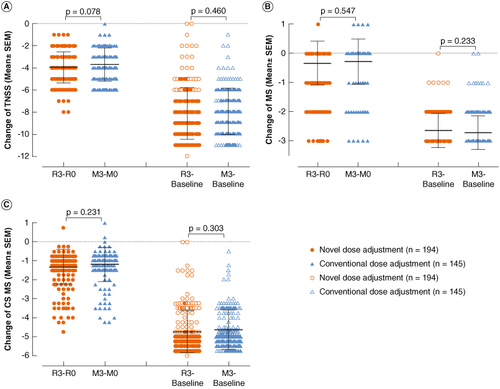
Figure 3. Comparison of changes in TNSS, MS and CSMS before and after treatment between the novel dose-adjustment protocol group and the conventional group. (A) Change in TNSS; (B) change in MS; (C) change in CSMS. R3-R0: The change at the end of 3-year treatment compared with the pretreatment baseline of the re-maintenance in the novel dose-adjustment protocol group; M3-M0: the change at the end of 3-year treatment compared with the baseline of maintenance treatment in the conventional group; R3-Baseline: the change from baseline to the end of 3-year treatment in the novel dose-adjustment protocol group; M3-Baseline: the change from baseline to the end of 3-year treatment in the conventional group.
CSMS: Combined symptom and medication score; MS: Medication score; TNSS: Total nasal symptom score.