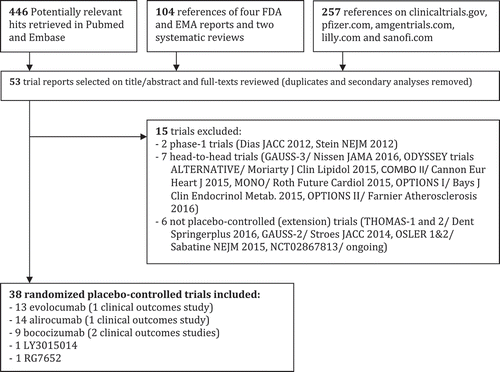
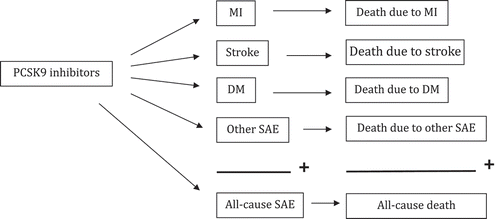
Figures & data
Table 1. Characteristics of included placebo-controlled PCSK9 inhibitor trials.
Figure 2. Risk for individual SAE, all-cause SAE, and all-cause mortality for PCSK9 inhibitors versus placebo in (a) lipid-lowering trials and (b) clinical outcome studies.
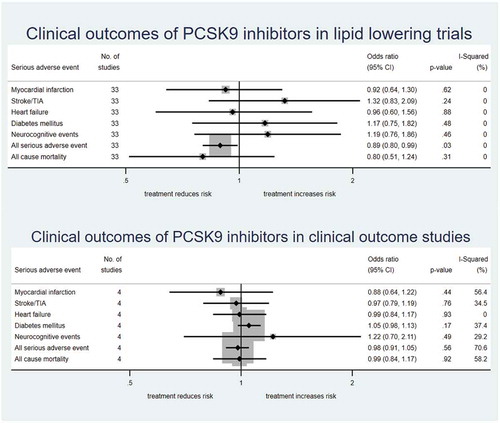
Table 2. Effect of PCSK9 inhibitors on clinical outcomes in randomized placebo-controlled trials according to data reported on ClinicalTrials.gov.
Table 3. Risk of clinical outcomes of evolocumab and alirocumab in randomized placebo-controlled trials according to data reported on ClinicalTrials.gov.