Figures & data
Figure 1. IL-6, its receptor system, and inhibitors at each stage of receptor complex formation. IL-6 has a four-bundle (from A to D) structure, interacting with the IL-6 receptor (IL-6 R) at site I and with gp130 at site II and site III. First, IL-6 binds to IL-6 R, the IL-6/IL-6 R complex binds to gp130, and finally a hexamer structure is formed. IL-6 signaling via membrane IL-6 R or soluble IL-6 R is called classic signaling or trans-signaling, respectively. Red arrows indicate signal transduction. Antibodies that inhibit the binding of IL-6 to IL-6 R, sgp130-Fc that inhibits the binding of IL-6/sIL-6 R complex to gp130, and antibodies that inhibit hexamer formation are surrounded by black squares. The antibodies listed in bold letters are approved for a variety of diseases.
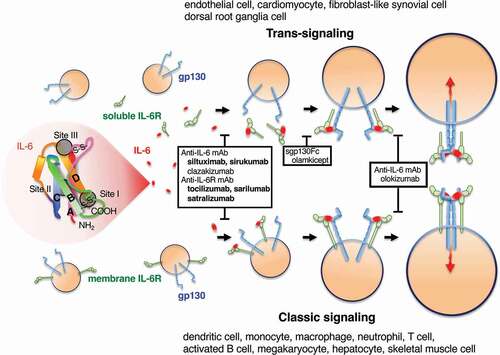
Table 1. Comparison of congenital loss of IL-6 receptor with IL-6 receptor inhibition therapy.
Table 2. Indications for IL-6–targeting therapy, approved diseases, phases of clinical trials, and case reports. Case reports excluded approved diseases and diseases for which randomized trials or case series showed the efficacy of IL-6–targeting therapy. TCZ: tocilizumab (anti–IL-6 R mAb); SAR: sarilumab (anti–IL-6 R mAb); STZ: satralizumab (anti–IL-6 R mAb); STX: siltuximab (anti–IL-6 mAb); SRK: sirukumab (anti–IL-6 mAb); CLZ: clazakizumab (anti–IL-6 mAb); OKZ: olokizumab; (anti–IL-6 mAb); OLA: olamkicept (soluble gp130-Fc fusion protein. TJ301); NMDAR; N-methyl-d-aspartate receptor; GVHD: graft-versus-host disease.
Figure 2. Diseases for which IL-6–targeting therapy has been approved, efficacy has been confirmed in randomized clinical trials, or efficacy has been reported in the case series can be divided into four categories: chronic inflammatory disease, acute inflammatory disease, autoantibody-induced disease, and interstitial lung disease.