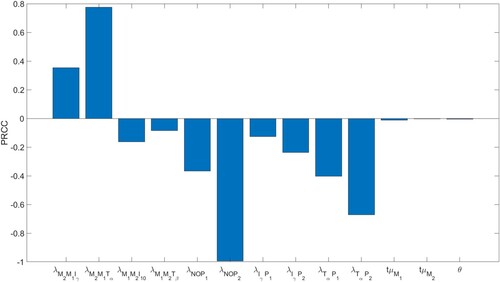
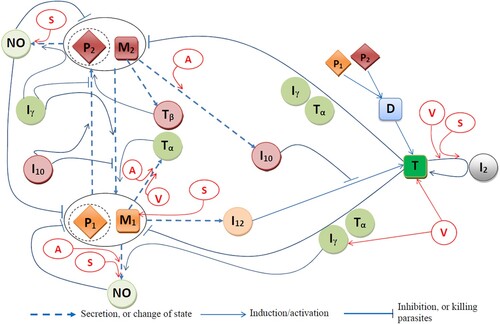
Figures & data
Table 1. List of variables of the model.
Table 2. Descriptions, values, and sources of the model parameters.
Table 3. Descriptions, values, and sources of the model parameters.
,
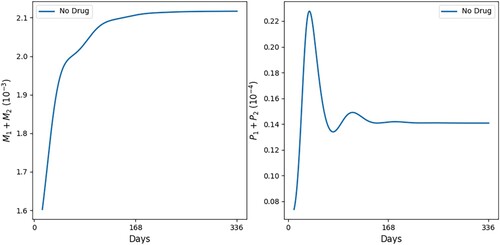
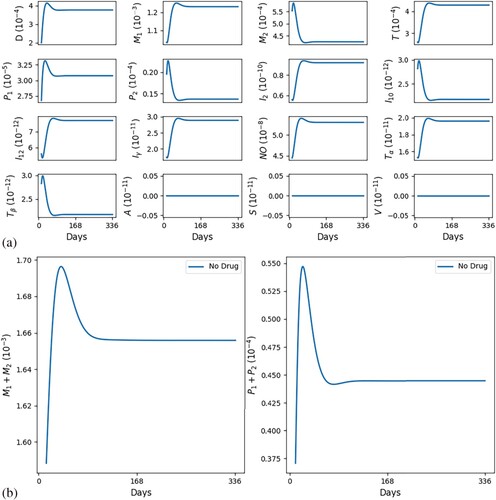
. (a) All variables with no self-healing and (b) all macrophages and parasites with no self-healing.
,
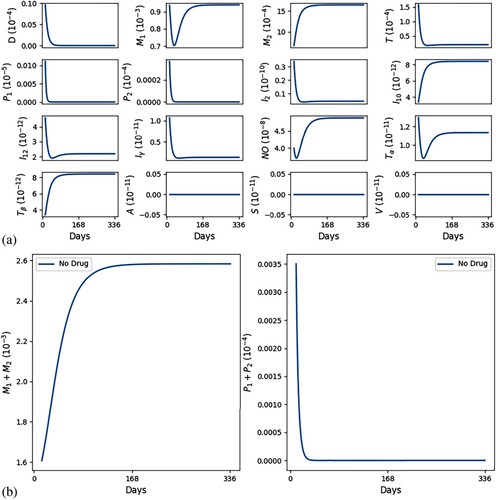
. (a) All variables with self-healing and (b) all macrophages and parasites with self-healing.
![Figure 5. Schedule for drug administration. As in [Citation65], shots of the vaccine LEISH-F1+MPL-SE adjuvant are given at Days 0, 28 and 56 for the LEISH-F1 and the MPL-SE adjuvant; meglumine antimoniate is administered daily, in cycles of 21 days, for the first 10 days followed by 11 days of rest.](/cms/asset/6670ea56-b9c9-4d61-8e36-b182c88df4cc/tjbd_a_2257746_f0005_ob.jpg)
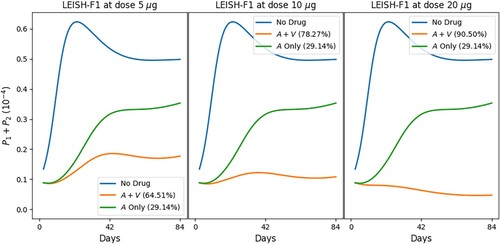
![Figure 6. Treatment of leishmaniasis with MA (A), vaccine LEISH-F1+MPL-SE (V) and MPL-SE adjuvant as in [Citation65]. The numbers in parentheses represent the recovery rates.](/cms/asset/44648a59-7122-4e81-ae19-a80d82906f85/tjbd_a_2257746_f0006_oc.jpg)
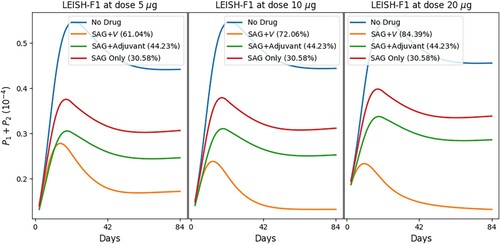
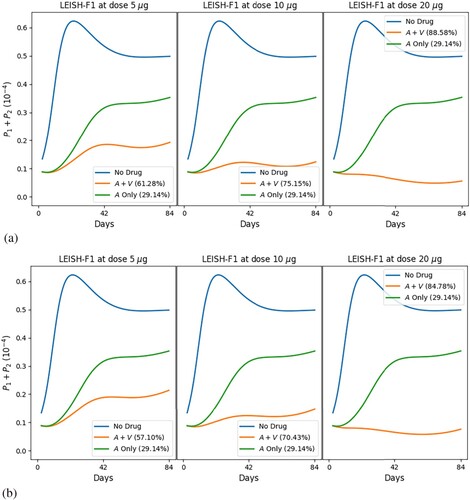
![Figure 7. Treatment of leishmaniasis with MA (A), vaccine LEISH-F1+MPL-SE (V) and MPL-SE adjuvant as in [Citation65]. Profiles of total parasites load with alternative doses of V . The numbers in parentheses represent the recovery rates.](/cms/asset/f49cd429-9c39-4ba7-a2f6-935342d276f9/tjbd_a_2257746_f0007_oc.jpg)
at day 84),
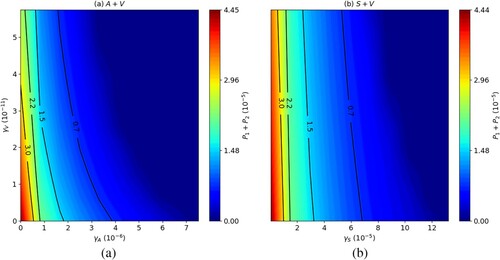
, combination of (a) MA with LEISH-F1+MPL-SE and (b) SAG with LEISH-F1+MPL-SE. The parasite loads are in units of g/cm
.