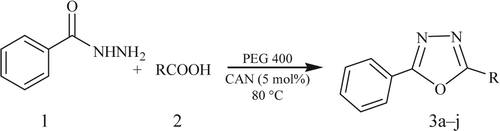
Figures & data
Figure 1. Synthesis of 2,5-disubstituted oxadiazoles in various solvents.a
aReaction conditions: carboxylic acid (1 mmol), benzhydrazide (1 mmol); temperature 80°C; solvent.
bIsolated and unoptimized yields.
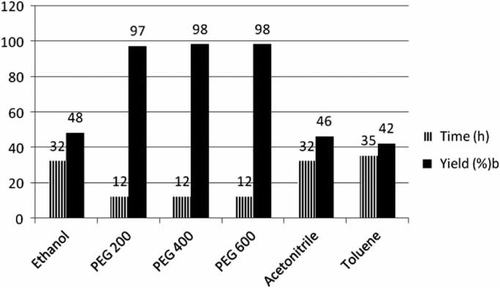
Figure 2. Catalytic activity evaluation of CAN for the synthesis of 2,5-disubstituted oxadiazoles.a
aReaction conditions: carboxylic acid (1 mmol), benzhydrazide (1 mmol); temperature 80°C; PEG 400.
bIsolated and unoptimized yields.
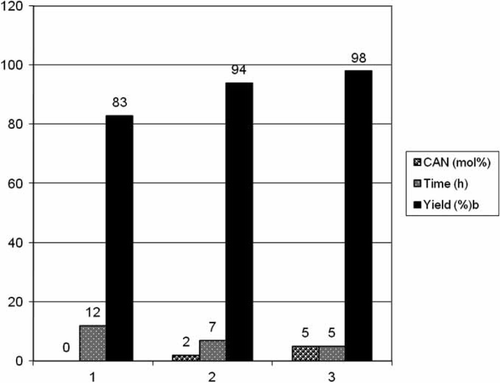
Table 1. Screening of various Lewis acids for the synthesis of 2,5-disubstituted oxadiazoles.a
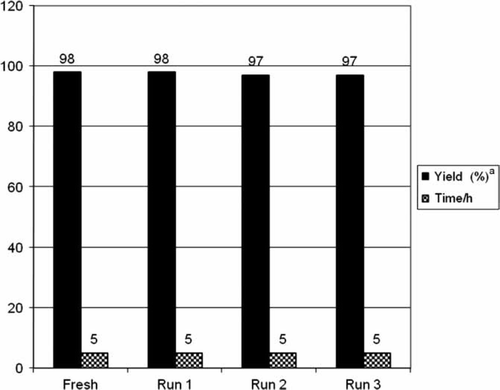
Figure 3. Recycling yields.
Note: Reaction conditions: carboxylic acid (1 mmol), benzhydrazide (1 mmol), CAN (5 mol%); solvent PEG 400; temperature 80°C.
aIsolated and unoptimized yields.