Figures & data
Figure 1. The degradation kinetics behavior of a typical polymer (red) and an ideal photochemically degradable polymer (blue). Note the tunable onset of degradation and the rapid degradation in the ideal polymer.
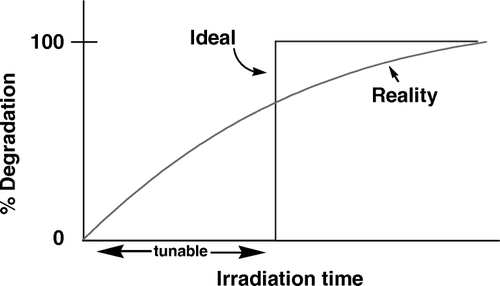
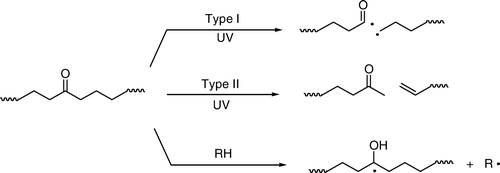
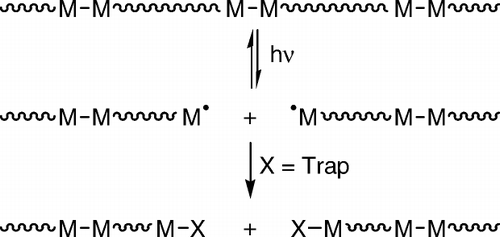

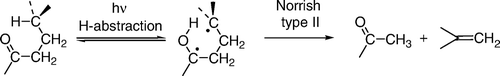
Figure 3. Plot of ln Φ versus T–1 for polymer 2.
Source: See Citation99, . With permission from Springer Science+Business Media.
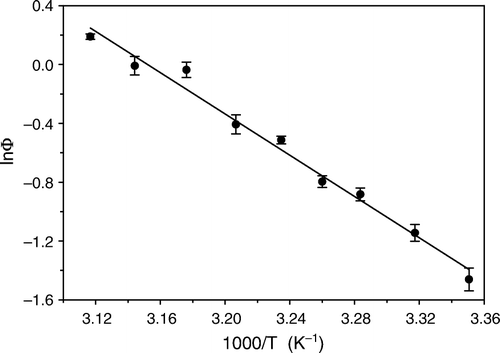
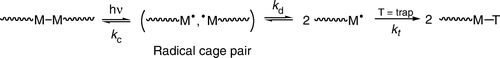
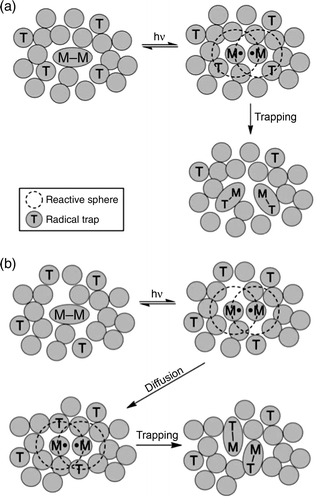
Figure 4. Illustration of the reaction of photogenerated metal radicals with trapping atoms in a solid-state matrix. In case (a) there is a trap in the reactive sphere of the metal radical and (b) the trap is initially outside of the reactive sphere.