Figures & data
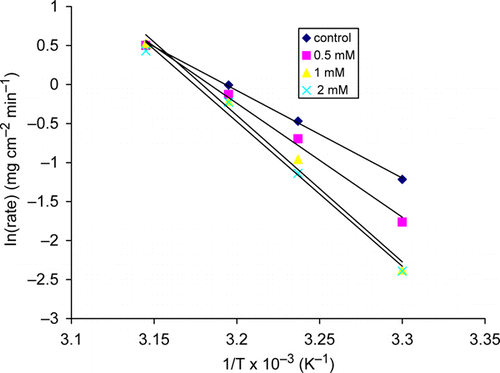
Figure 1. Variation of weight loss with thiamine hydrochloride concentration for copper in 2.5 M HNO3 at 30°C.
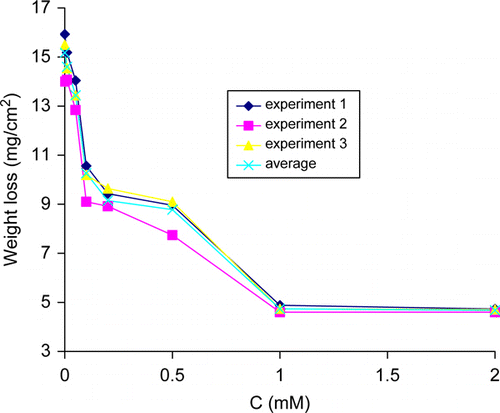
Table 1. Percentage inhibition efficiency, corrosion rate, and surface coverage of thiamine of thiamine hydrochloride on copper at 30°C 30 min immersion period.
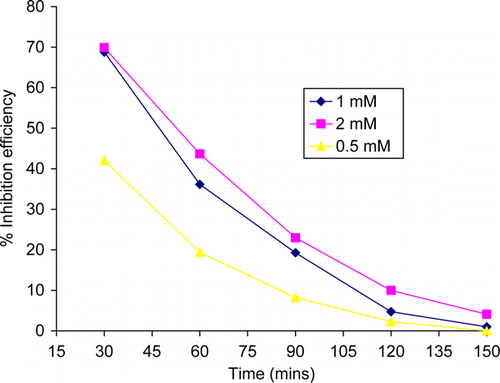
Figure 2. Variation of percentage inhibition efficiency of thiamine hydrochloride with immersion period.
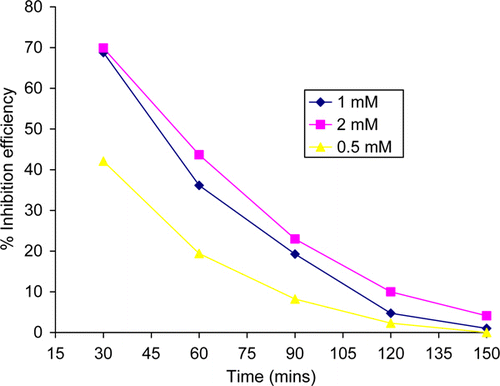
Figure 3. Langmuir adsorption plot for copper in 2.5 M HNO3 solution containing thiamine hydrochloride at 30°C.
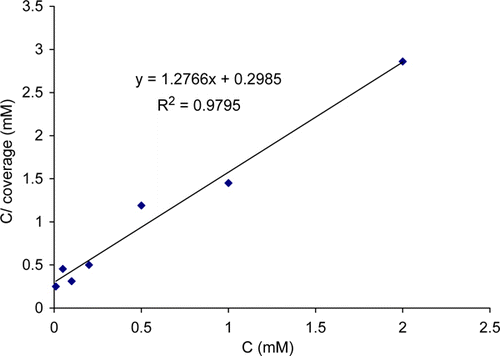
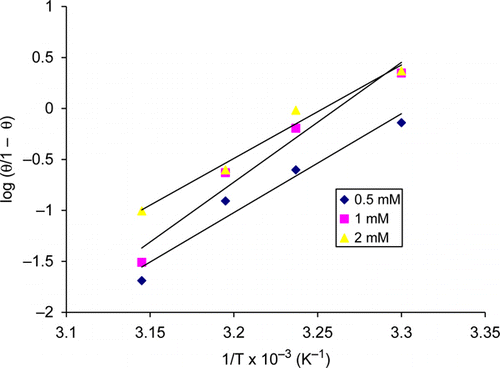
Figure 5. Plot of log (θ/1 – θ) versus 1/T at concentrations of 0.5, 1, and 2 mM thiamine hydrochloride.