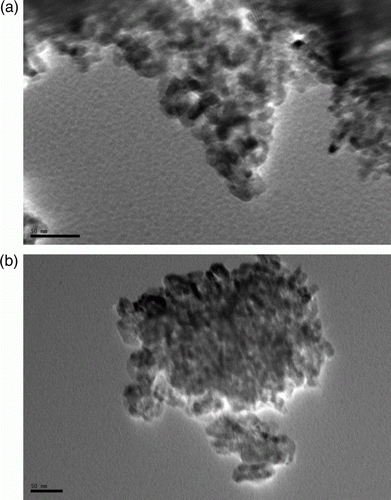
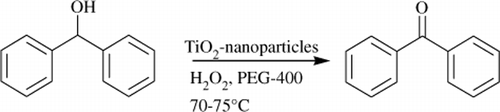Figures & data
Table 1. Oxidation of alcohols using different nanocrystalline metal oxides.a
Table 2. Screening of solvent for oxidation reaction.
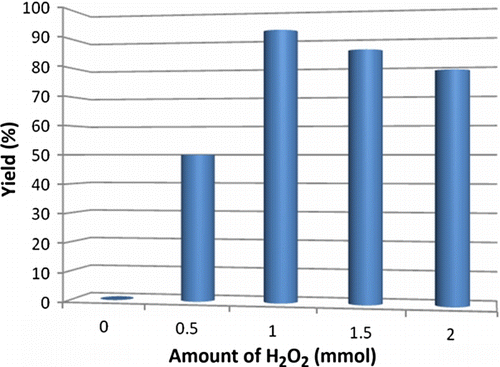
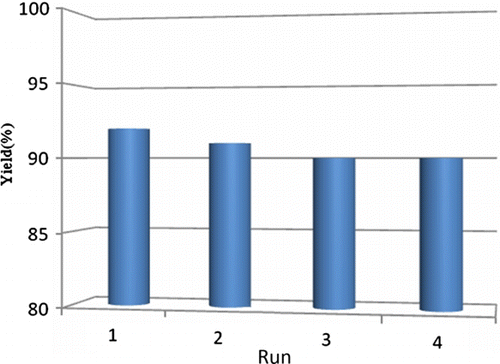
Figure 2. Optimization of concentration of H2O2. Reaction conditions: diphenylcarbinol (1 mmol), hydrogen peroxide (1 mmol) and 10 mol% nano TiO2 (50±2) nm; PEG-400; temperature 70–75°C. Isolated yields.