Figures & data
Figure 1. TEM image of silica nanoparticles used as catalysts for the one step, three-component synthesis of highly substituted pyridines (13). Reprinted with permission from Elsevier © 2009.
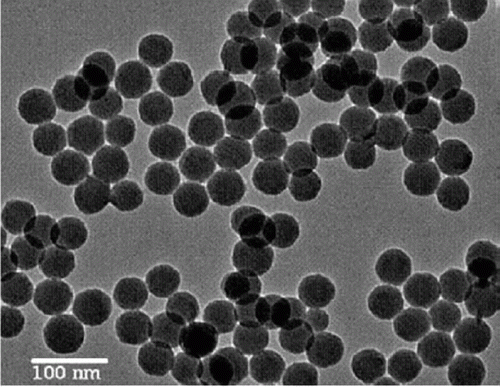
Figure 2. (a) SEM and (b) TEM images of the titania nanoparticles (16). Reprinted with permission from Elsevier © 2009.
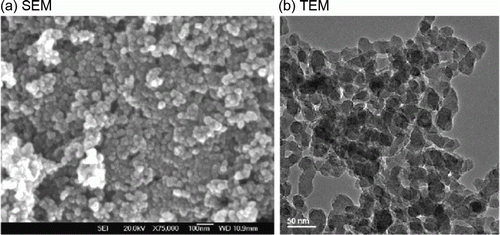
Figure 3. SEM images of palladium nanoparticles of different shapes that are used as catalysts for the carbon–carbon coupling reactions (21). Reproduced by permission of the Royal Society of Chemistry.
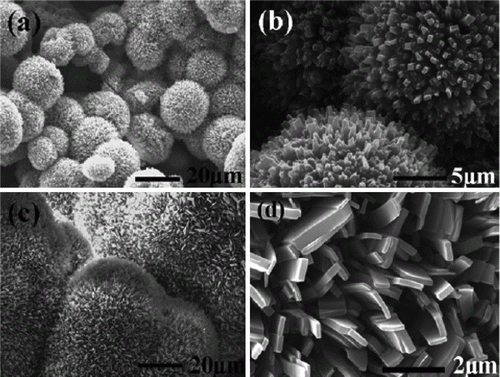
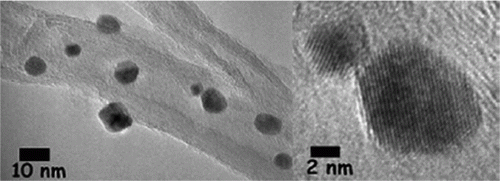
Figure 4. TEM image of the palladium nanoparticles prepared using the biomimetic synthesis method (22). Reprinted with permission from American Chemical Society © 2009.
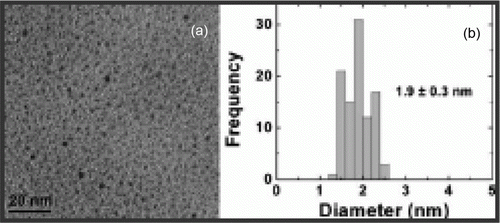
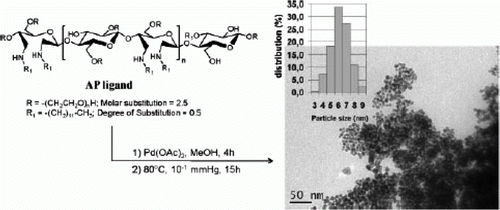
Figure 5. TEM images of the palladium nanoparticles after removing ionic liquid in solution, size distribution histogram and electron diffraction patterns (27). Reproduced by permission from the Royal Society of Chemistry.
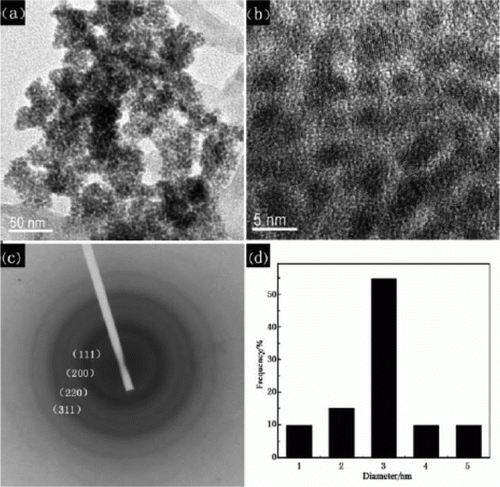
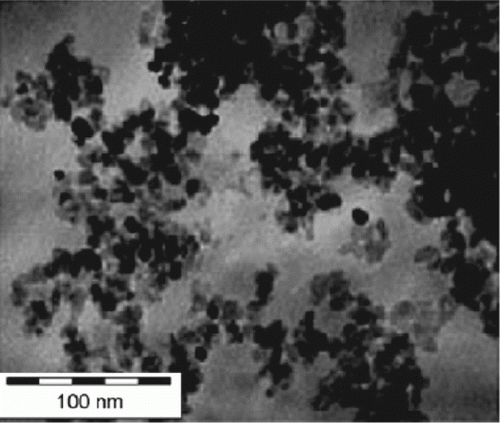
Figure 6. TEM image of platinum nanoparticles that are stabilized in the presence of ionic liquids (29). Reprinted with permission from Elsevier © 2009.
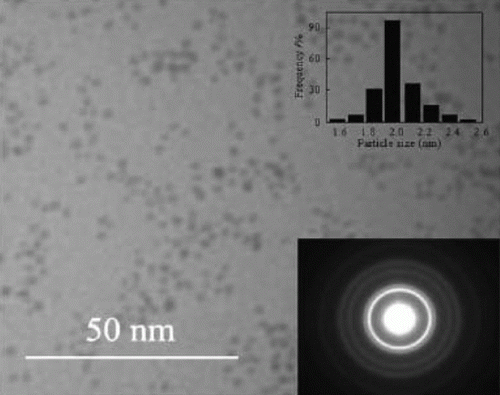
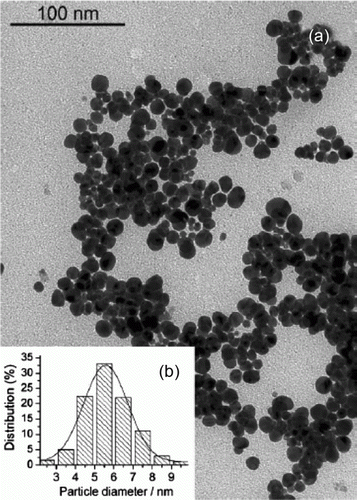
Figure 7. SEM and TEM images of the gold nanoparticles adsorbed onto mesoporous silica (32). Reprinted with permission from American Chemical Society © 2009.
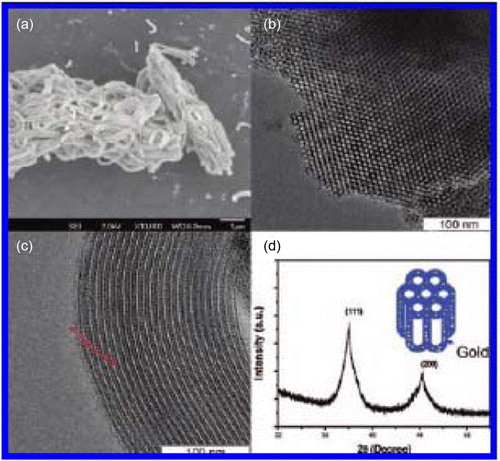
Figure 8. Silver nanoparticles that are synthesized using the green photocatalytic synthesis method (35). Reproduced by permission of the Royal Society of Chemistry.
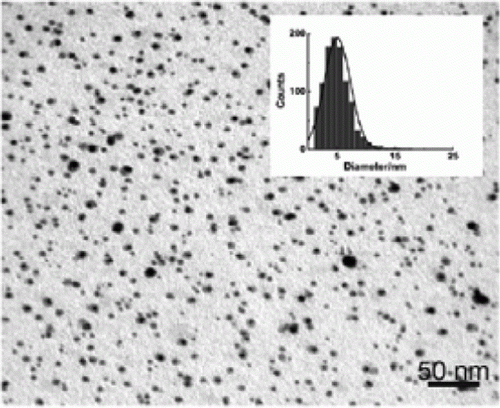
Figure 9. TEM images of the gold-palladium nanoparticle synthesized in the absence of organic liquids as stabilizers (38). Reproduced by permission of the Royal Society of Chemistry.
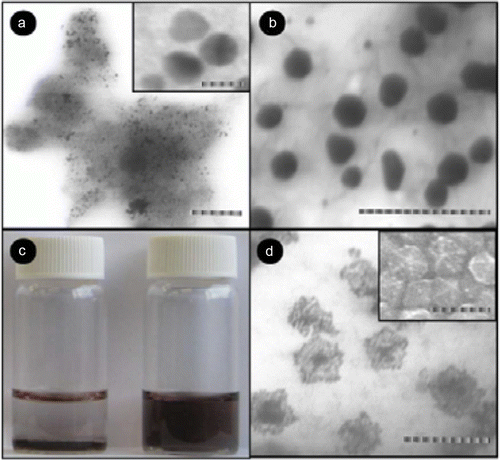
Figure 10. TEM image and size distribution of iron nanoparticles that are used as catalysts for alkene and alkyne hydrogenation reactions (45). Reproduced by permission of the Royal Society of Chemistry.
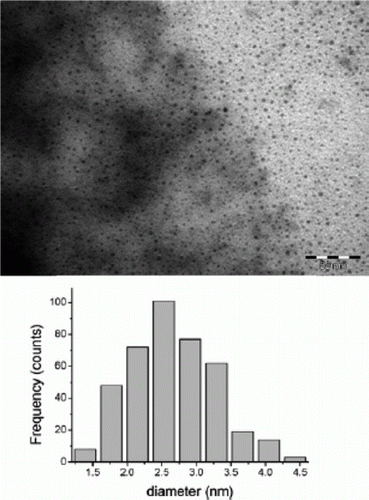
Figure 11. Rhodium nanoparticles synthesized in water and used as catalysts for hydrogenation of different heteroaromatic compounds (47). Reprinted.
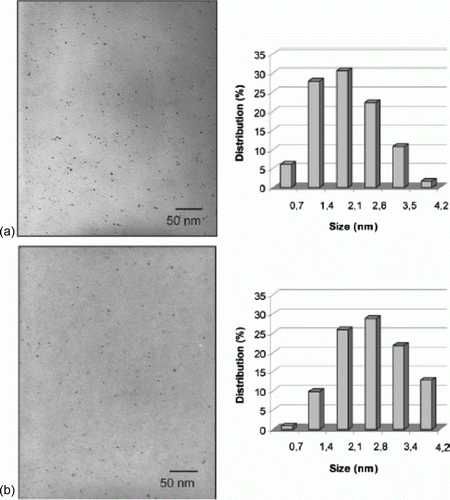
Figure 12. TEM image and size distribution of the gold nanoparticles supported on hydrotalcite (52). Reproduced by permission of the Royal Society of Chemistry.
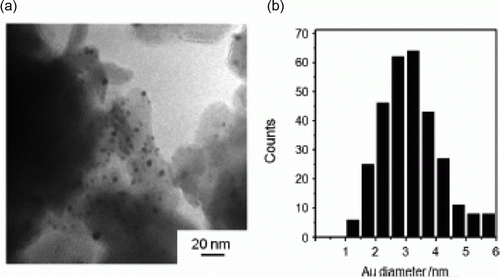
Figure 13. TEM image of the palladium nanoparticles formed using PEG-1000 as the stabilizer before oxidation of alcohols (a) and after oxidation (b) (53). Reproduced with permission from John Wiley and Sons.
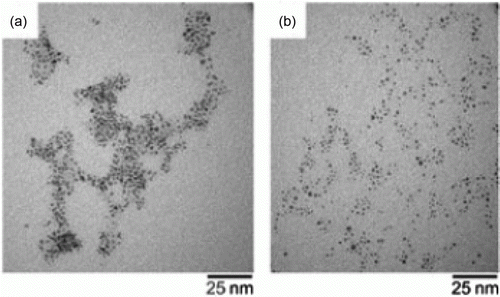
Figure 14. TEM images of palladium nanoparticles before and after oxidation of secondary alcohols (58). Reprinted with permission from Elsevier © 2010.
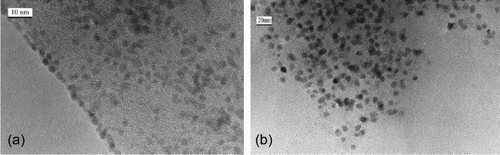
Figure 15. TEM images of the palladium nanoparticles supported on graphite oxide (66). Reprinted with permission from Elsevier © 2010.
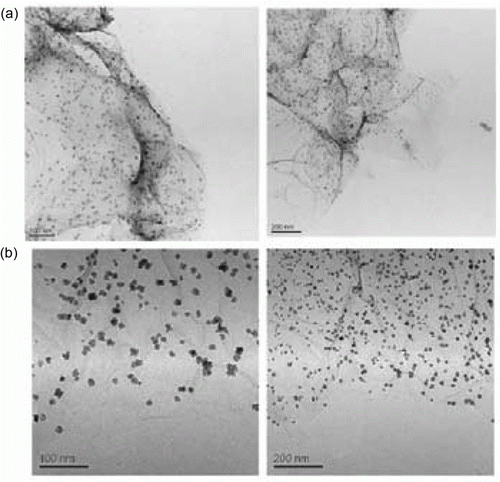
Figure 16. TEM image of palladium nanoparticles used as catalysts for the Sonogashira coupling reaction (69). Reproduced with permission from John Wiley and Son.
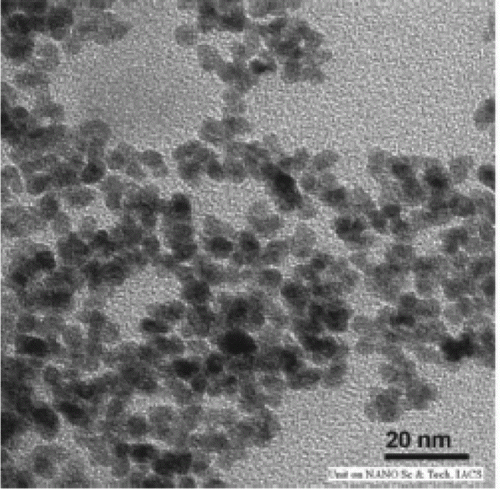
Figure 17. High-resolution TEM image of titania support and the rhodium nanoparticles adsorbed onto the titania support (46). Reproduced by permission of the Royal Society of Chemistry.
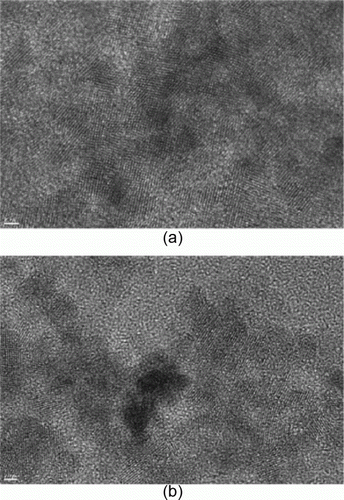
Figure 18. TEM images of palladium nanoparticles used as catalysts for the Ullmann type homocoupling reactions (72). Reprinted with permission from American Chemical Society © 2010.
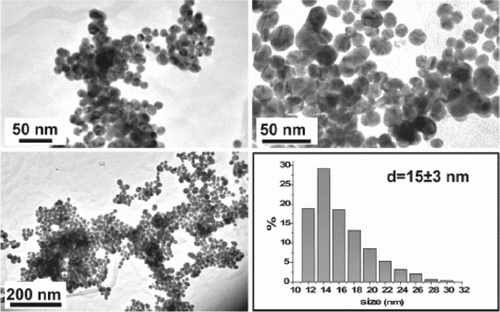
Figure 19. TEM image, size distribution, and electron diffraction patterns of FePt nanoparticles (73). Reproduced by permission of the Royal Society of Chemistry.
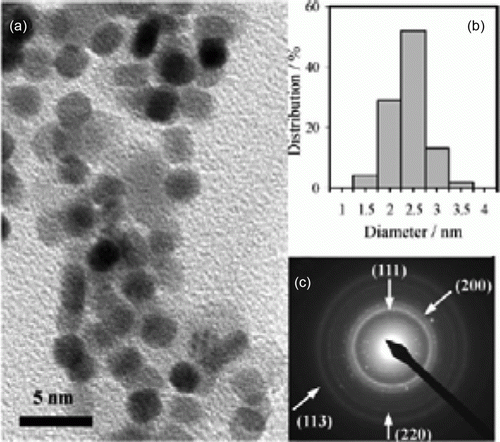
Figure 20. SEM image of the nickel nanocatalysts before and after the sixth cycle of the Heck cross-coupling reaction (75). Reproduced by permission of the Royal Society of Chemistry.
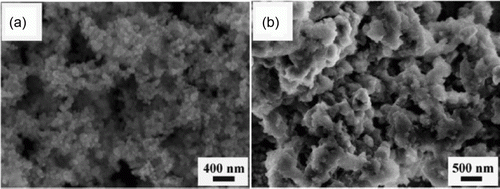
Figure 21. Palladium nanoparticles adsorbed onto carbon nanotubes (78). Reproduced by permission of the Royal Society of Chemistry.