Open access
747
Views
6
CrossRef citations to date
0
Altmetric
RESEARCH LETTERS
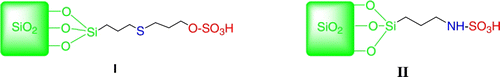
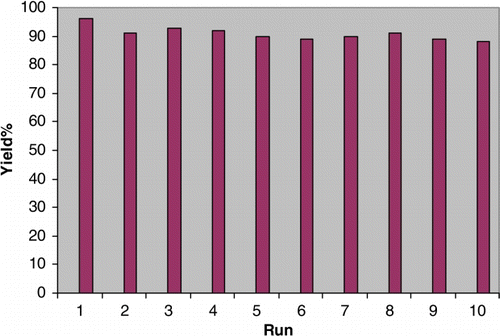
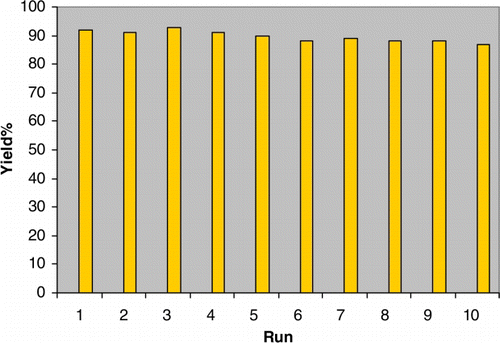
Silica immobilized sulfuric acid ([3-(propyl)sulfanyl]propyl]ester and N-propylsulfamic acid as recyclable catalysts for chemoselective synthesis of 1,1-diacetates
Sedigheh Pargaleh Brojeni
Chemistry Department, Islamic Azad University, Gachsaran Branch, Gachsaran, Iran
, Mojtaba Baghernejad
Chemistry Department, Islamic Azad University, Gachsaran Branch, Gachsaran, Iran
, Dariush Saberi
Department of Chemistry, Faculty of Sciences, Persian Gulf University, Bushehr, 75169, Iran
& Khodabakhsh Niknam
Department of Chemistry, Faculty of Sciences, Persian Gulf University, Bushehr, 75169, IranCorrespondence[email protected]
Pages 69-75
|
Received 18 May 2010, Accepted 02 Jun 2012, Published online: 14 Jan 2013
Related research
People also read lists articles that other readers of this article have read.
Recommended articles lists articles that we recommend and is powered by our AI driven recommendation engine.
Cited by lists all citing articles based on Crossref citations.
Articles with the Crossref icon will open in a new tab.