Figures & data
Figure 1. Structures of chemiluminescent acridinium dimethylphenyl ester labels containing N-sulfopropyl groups in the acridinium ring that are used in automated immunoassays in Siemens Healthcare Diagnostics' ADVIA:Centaur® systems Citation1–3.
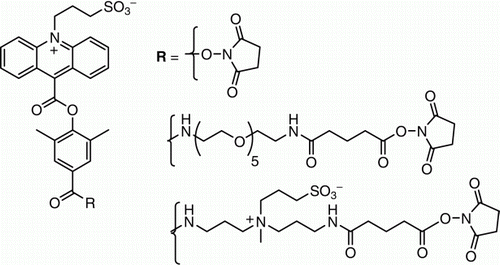
Figure 2. N-Alkylation of acridine methyl ester 2a with 1,3-propane sultone Citation14. The reaction proceeds via a charged transition state and is facilitated by polar solvents such as the ILs [BMIM][PF6] and [BMIM[BF4].
![Figure 2. N-Alkylation of acridine methyl ester 2a with 1,3-propane sultone Citation14. The reaction proceeds via a charged transition state and is facilitated by polar solvents such as the ILs [BMIM][PF6] and [BMIM[BF4].](/cms/asset/a86fb901-4d20-48a6-975e-235aaa9c8375/tgcl_a_768708_o_f0002g.jpg)
Figure 3. Synthetic scheme for the synthesis of acridan esters and their N-alkylation reactions with sodium 3-bromopropane sulfonate in [BMIM][BF4]. The acridan N-alkylation reaction also has a charged transition state and is facilitated in the IL [BMIM][BF4]. Reagents: (a) Thionyl chloride, ROH; (b) p-toluenesulfonyl chloride, pyridine; (c) picoline-borane, tetrahydrofuran, 10% HCl; (d) sodium 3-bromopropane sulfonate, potassium carbonate, [BMIM][BF4]; (e) air or oxygen; (f) 1-2 M HCl; (g) TSTU, diisopropylethylamine, DMF.
![Figure 3. Synthetic scheme for the synthesis of acridan esters and their N-alkylation reactions with sodium 3-bromopropane sulfonate in [BMIM][BF4]. The acridan N-alkylation reaction also has a charged transition state and is facilitated in the IL [BMIM][BF4]. Reagents: (a) Thionyl chloride, ROH; (b) p-toluenesulfonyl chloride, pyridine; (c) picoline-borane, tetrahydrofuran, 10% HCl; (d) sodium 3-bromopropane sulfonate, potassium carbonate, [BMIM][BF4]; (e) air or oxygen; (f) 1-2 M HCl; (g) TSTU, diisopropylethylamine, DMF.](/cms/asset/8ec543d0-6604-4d12-a832-74fbcbb45a2b/tgcl_a_768708_o_f0003g.jpg)
Table 1. N-Alkylation of acridan dimethylphenyl esters with sodium 3-bromopropane sulfonate in [BMIM][BF4].
Table 2. N-Alkylation of acridine ester 2a with sodium 3-bromopropane sulfonate in ionic liquids.
Figure 4. HPLC traces. Upper panel: analysis of the reaction mixture from N-alkylation (, steps d and e) on a gram scale of acridan isopropyl ester 3b with sodium 3-bromopropane sulfonate in [BMIM][BF4] followed by oxidation. Compound eluting at 6.4 minutes is product 5b and compound eluting at 10.2 minutes is acridine isopropyl ester 2b. Small peaks eluting at 4.4 minutes and 7.3 minutes are the corresponding carboxylic acids of 5b and 2b, respectively; middle panel: analysis of the product 5b following purification by column chromatography; lower panel: analysis of the N-sulfopropyl acridinium carboxylic acid 6 obtained by hydrolysis of the isopropyl ester 5b.
![Figure 4. HPLC traces. Upper panel: analysis of the reaction mixture from N-alkylation (Figure 3, steps d and e) on a gram scale of acridan isopropyl ester 3b with sodium 3-bromopropane sulfonate in [BMIM][BF4] followed by oxidation. Compound eluting at 6.4 minutes is product 5b and compound eluting at 10.2 minutes is acridine isopropyl ester 2b. Small peaks eluting at 4.4 minutes and 7.3 minutes are the corresponding carboxylic acids of 5b and 2b, respectively; middle panel: analysis of the product 5b following purification by column chromatography; lower panel: analysis of the N-sulfopropyl acridinium carboxylic acid 6 obtained by hydrolysis of the isopropyl ester 5b.](/cms/asset/1fb0b2c9-f995-413c-8885-7e47bd76aa82/tgcl_a_768708_o_f0004g.jpg)