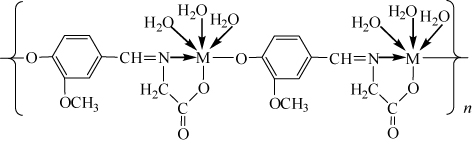
Figures & data
Table 1. Elemental analyses results and the melting point of the ligand and the complexes.
![Figure 1. X-ray powder diffraction patterns of the complexes [Zn(C10H9O4N)(H2O)3] (a) and [Ni(C10H9O4N)(H2O)3]·1.5H2O (b).](/cms/asset/324c9102-33a0-4109-8e59-7809090fe306/tgcl_a_927008_f0001_b.jpg)
Table 2. Experimental data and calculated results for powder X-ray diffraction pattern of the complex [Zn(gly-van)(H2O)3] (monoclinic system: a = 0.6807 nm, b = 1.3818 nm, c = 1.2011 nm, and β = 95.80°).
Table 3. Experimental data and calculated results for powder X-ray diffraction pattern of the complex [Ni(gly-van)(H2O)3]·1.5H2O (monoclinic system: a = 0.7457 nm, b = 1.3331 nm, c = 1.2560 nm, and β = 91.89°).
![Figure 2. Infrared spectra of the complexes [Zn(C10H9O4N)(H2O)3] (a) and [Ni(C10H9O4N)(H2O)3]·1.5H2O (b).](/cms/asset/ee5dd1f5-7002-4679-b127-c57a848533bb/tgcl_a_927008_f0002_b.jpg)
![Figure 3. TG–DSC curves of the complexes [Zn(C10H9O4N)(H2O)3] (a) and [Ni(C10H9O4N)(H2O)3]·1.5H2O (b).](/cms/asset/b1b6c6e2-d41d-41b4-93fe-c6ff4ee22bd7/tgcl_a_927008_f0003_b.jpg)