Figures & data
![Scheme 1. Synthesis and reduction of 2-aryl imidazo[1,2-a]azines-3-carbaldehyde 3 and 4. Reaction conditions: (a) N2, anh DMF/POCl3/0–100°C, 1 h then aq. KOH, (b) ethyl alcohol, NaBH4/0.1 N NaOH/rt, then 0.1 N HCL, and (c) electrochemical reduction, copper electrodes, H2O/electric current, e-/rt](/cms/asset/d9f4f546-522a-4850-89b6-ccac111e52d6/tgcl_a_946973_f0001_b.jpg)
Table 1. Yields of 2-aryl-3-hydroxymethyl imidazo[1,2-a]pyridines 5 by reduction with NaBH4 and by electrolysis of corresponding 2-aryl imidazo[1,2-a]pyridine-3-carbaldehyde 3.
Table 2. Yields of 2-aryl-3-hydroxymethyl imidazo[1,2-a]pyrimidines 6 by reduction with NaBH4 and by electrolysis of corresponding 2-aryl imidazo[1,2-a]pyrimidine-3-carbaldehyde 4.
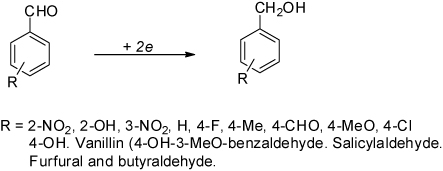
Table 3. Synthesis of primary alcohols from diverse aldehydes by electrolysis.
Supplemental material

![Scheme 3. Synthesis and attempted electrolytic reduction of 2-arylimidazo[2,1-b]thiazole-3-carbaldehyde derivatives.](/cms/asset/406470e1-ca0a-4720-bf2d-fb13c1253abb/tgcl_a_946973_f0003_b.jpg)
![Scheme 4. Electrosynthesis of 2-aryl-3-hydroxymethyl imidazo[1,2-a]azines.](/cms/asset/eed9dac4-bf80-4142-bdee-68c6bbbfc2d9/tgcl_a_946973_f0004_b.jpg)