Figures & data
Figure 1. (a) Determination of point of zero charge of P. cruentum and (b) FTIR spectra of unloaded and Hg(II)-loaded P. cruentum.
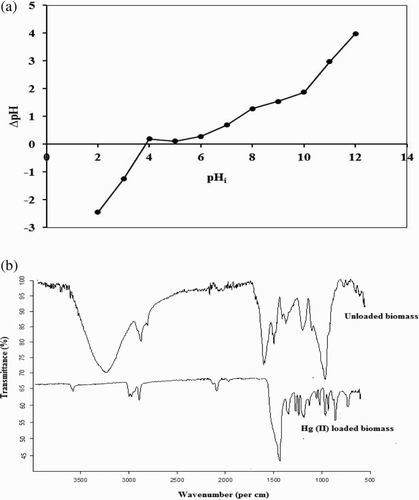
Figure 2. Effect of biosorbent dosage on biosorption studies of Hg(II) by P. cruentum (Co = 10 mg/L, contact time = 1440 min, solution pH).
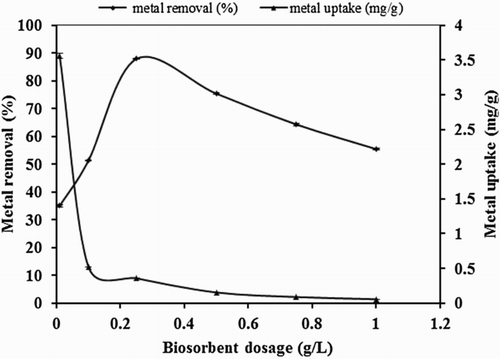
Figure 3. Effect of pH on biosorption studies of Hg(II) by P. cruentum (Co = 10 mg/L, biosorbent dosage = 0.25 g/L, contact time = 1440 min). Inset graph indicates the speciation of Hg(II) with pH.
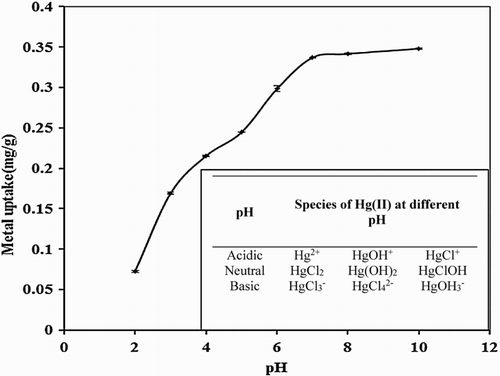
Figure 4. Effect of contact time on biosorption studies of Hg(II) by P. cruentum (Co = 10 mg/L, biosorbent dosage = 0.25 g/L, pH = 7). Inset graph shows the fitting of PFO and pseudo-second-order kinetic models.
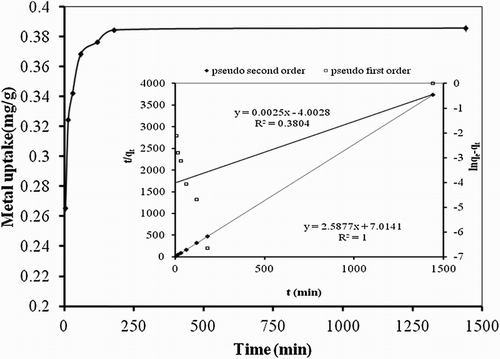
Table 1. PFO and second-order kinetics parameters for the biosorption of Hg(II) on P. cruentum.
Figure 5. Effect of initial metal concentration on biosorption studies of Hg(II) by P. cruentum (biosorbent dosage = 0.25 g/L, pH = 7, contact time = 120 min).
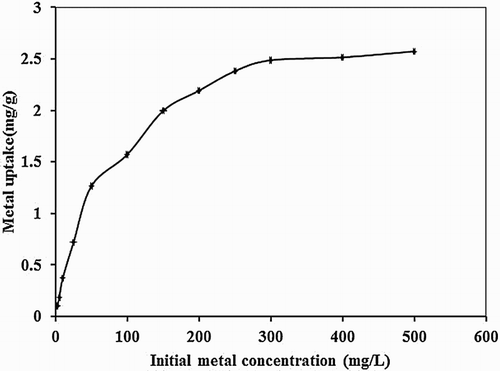
Table 2. Parameters for equilibrium models for the biosorption of Hg(II) onto P. cruentum.
Figure 6. Effect of temperature studies on biosorption studies of Hg(II) by P. cruentum (biosorbent dosage = 0.25 g/L, pH = 7, contact time = 120 min). Inset graph shows thermodynamics of the process.
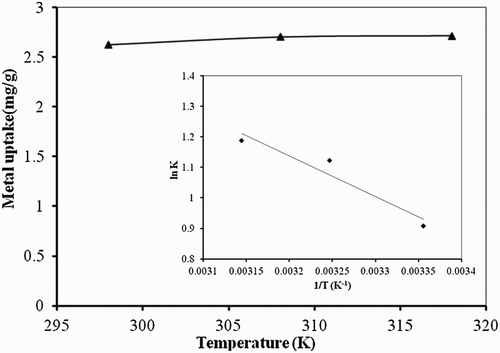
Table 3. Thermodynamics parameters for Hg(II) biosorption on P. cruentum.
Table 4. Comparison of Hg(II) biosorption efficiency of P. cruentum with other red algal species.
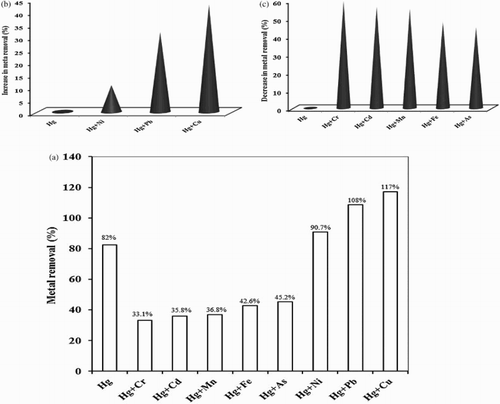
Figure 7. Effect of foreign metal ions in the binary system on biosorption studies of Hg(II) by P. cruentum (a) (Co(each) = 10 mg/L, biosorbent dosage = 0.25 g/L, pH = 7, contact time = 120 min) (b) represents the increased removal percentage and (c) indicates the decreased removal percentage due to the presence of interfering metal ions.