Figures & data
Figure 1. Effect of M. sativa concentration and temperature on the weight loss tests for 1018 carbon steel in 0.5 M H2SO4.
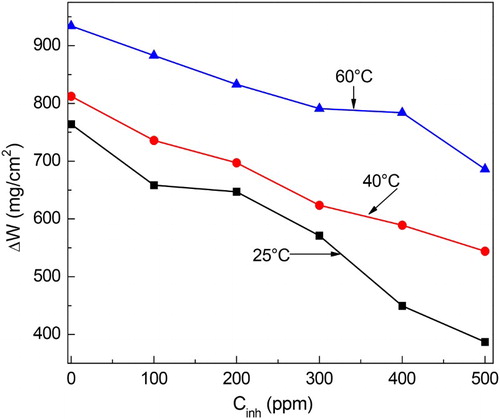
Figure 2. Effect of M. sativa concentration and temperature on the inhibitor efficiency value for 1018 carbon steel in 0.5 M H2SO4.
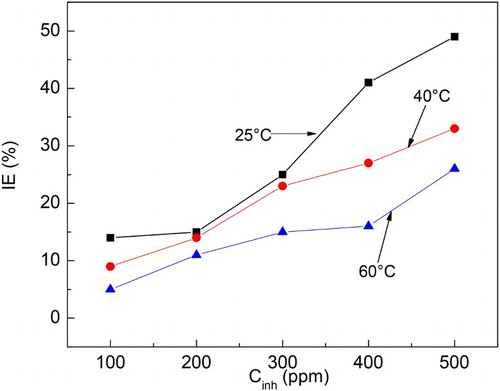
Figure 3. Frumkin type of adsorption isotherm for 1018 carbon steel in 0.5 M H2SO4 with additions of M. sativa.
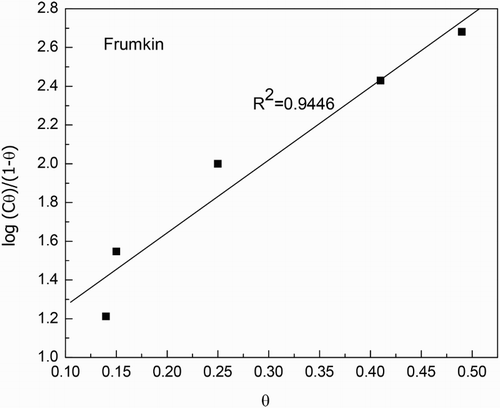
Table 1. Electrochemical parameters for polarization curves at 25°C.
Figure 4. Effect of M. sativa concentration on the polarization curves for 1018 carbon steel in 0.5 M H2SO4 at 25°C.
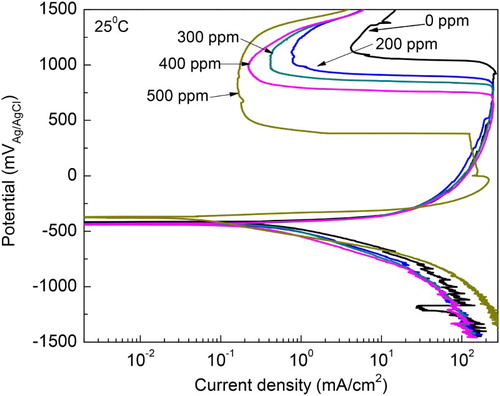
Figure 5. Effect of M. sativa concentration on the polarization curves for 1018 carbon steel in 0.5 M H2SO4 at 40°C.
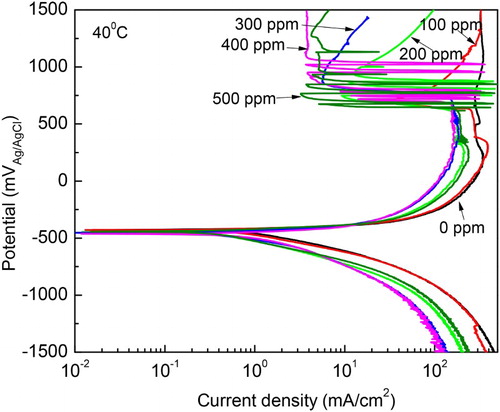
Figure 6. Effect of M. sativa concentration on the polarization curves for 1018 carbon steel in 0.5 M H2SO4 at 60°C.
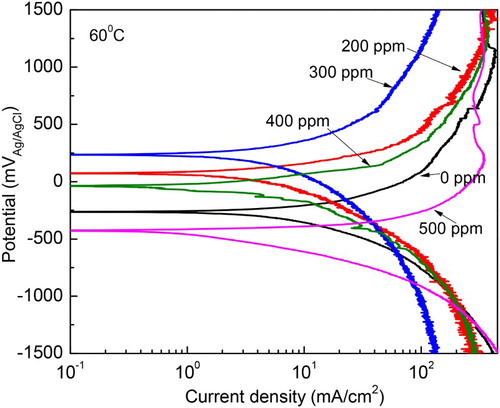
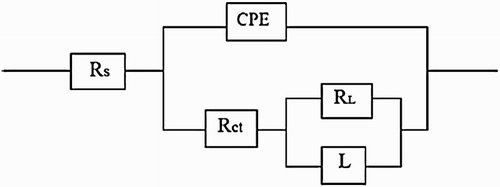
Figure 7. Effect of M. sativa concentration on the (a) Nyquist and (b) Bode plots for 1018 carbon steel in 0.5 M H2SO4 at 25°C.
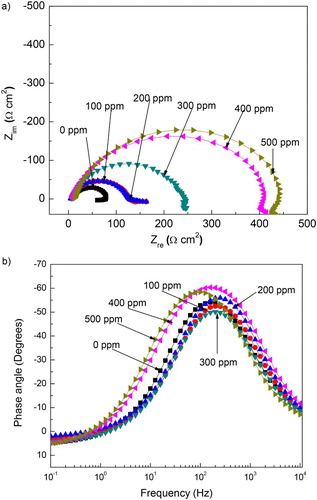
Figure 9. Variation of the Nyquist curves with time for 1018 carbon steel in 0.5 M H2SO4 at 25°C with the addition of 500 ppm of M. sativa.
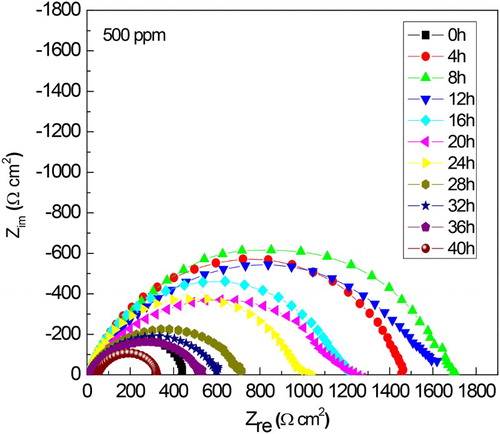
Figure 10. Variation of the Rp and Cdl values with time for 1018 carbon steel in 0.5 M H2SO4 at 25°C with the addition of 500 ppm of M. sativa.
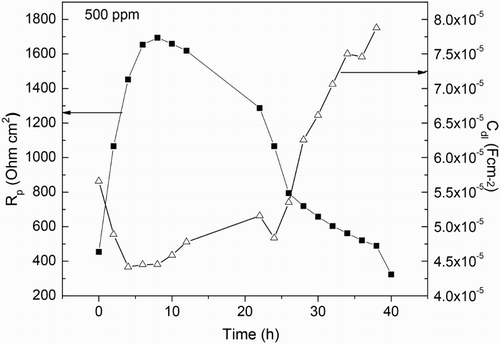
Figure 11. Effect of M. sativa concentration on the Nyquist plots for 1018 carbon steel in 0.5 M H2SO4 at 40°C.
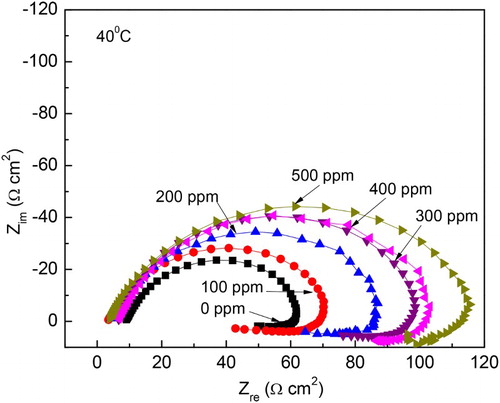
Figure 12. Variation of the Rp values with time for 1018 carbon steel in 0.5 M H2SO4 at 25°C with the addition of 0 and 500 ppm of M. sativa.
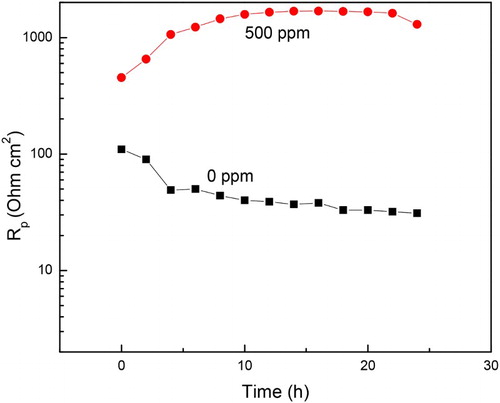
Figure 13. Variation of the inhibitor efficiency value with time for 1018 carbon steel in 0.5 M H2SO4 at 25°C with 500 ppm of M. sativa.
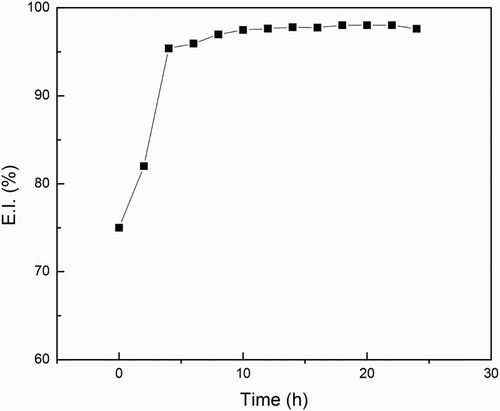
Figure 14. Effect of M. sativa concentration on the Nyquist plots for 1018 carbon steel in 0.5 M H2SO4 at 60°C.
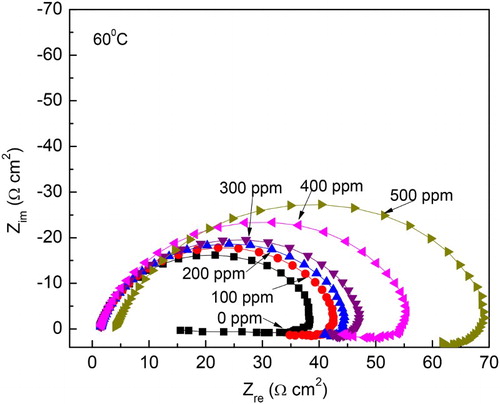
Figure 15. SEM micrographs of corroded 1018 steel specimens in uninhibited 0.5 M H2SO4 (a, c, e); and with addition of 500 ppm of M. sativa extract (b, d, f) at 25°C (a, b), 40°C (c, d) and 60°C (e, f).
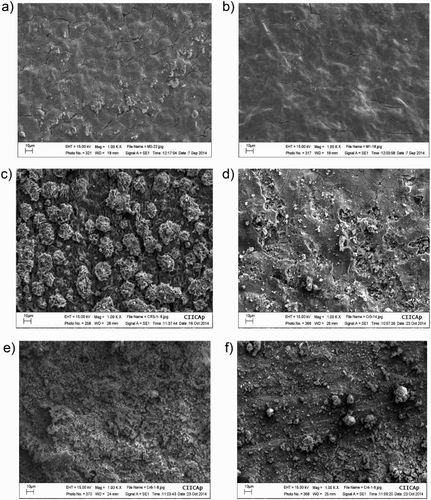
Figure 16. EDX analysis of corroded 1018 steel specimens in 0.5 M H2SO4 containing (a) 0 and (b) 500 ppm of M. sativa extract at 25°C.
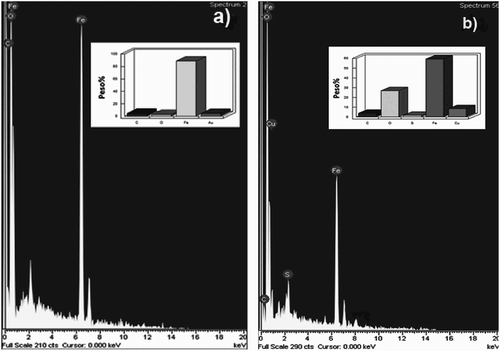
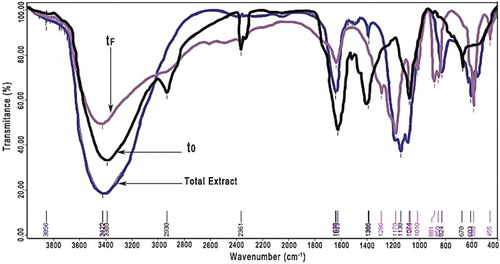
Figure 17. Infrared spectroscopy of M. sativa (pure extract or total extract), before (to) and after the corrosion test (tf).