Figures & data
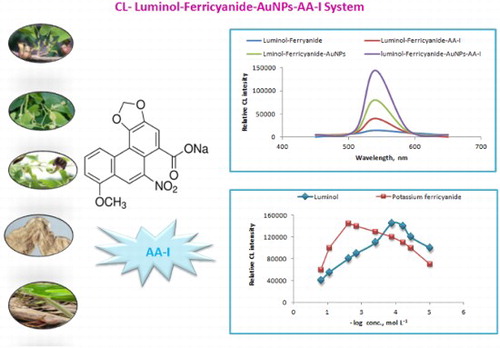
Figure 4. Effect of luminol and potassium ferricyanide concentration on CL intensity, for luminol concentration (1.5 mL of AuNPs solution and potassium ferricyanide 1.0 × 10−2 mol L−1) and for potassium ferricyanide concentration (1.5 mL of prepared AuNPs and luminol 2.0 × 10−4 mol L−1) with AA-I 25 g mL−1.
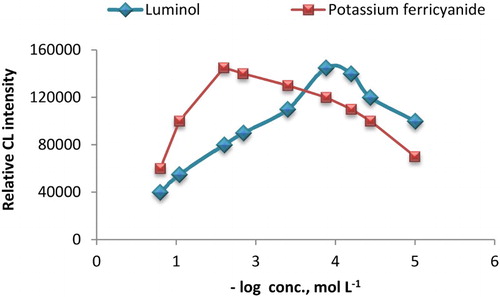
Figure 6. Effect of sodium hydroxide, ammonium hydroxide, sodium carbonate and sodium bicarbonate concentration on CL intensity of luminol-potassium ferricyanide system (1.5 mL of AuNPs, potassium ferricyanide 1.0 × 10−2 mol L−1 and luminol 2.0 × 10−4 mol L−1).
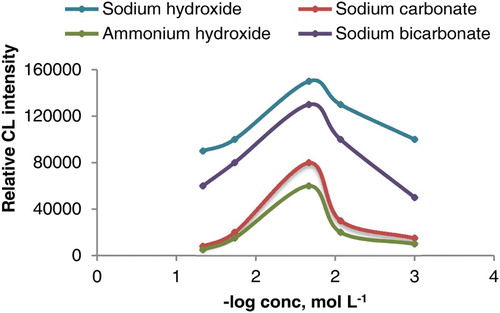
Figure 7. The influence of flow rate on the relative CL intensity. Conditions; 50 µL of 2.0 × 10−4 mol L−1 luminol; 30 µL of AuNPs and 30 µL of 1.0 × 10−2 mol L−1 potassium ferricyanide.
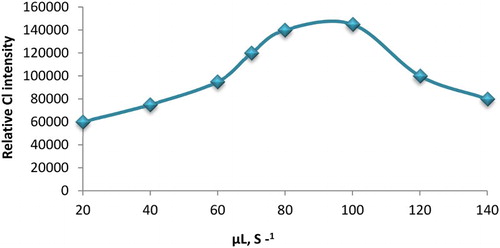
Table 1. The control program of AuNPs-luminol-potassium ferricyanide SIA-CL detection of AA-I.
Table 2. Performance data obtained from the determination of AA-I using AuNPs-luminol-potassium ferricyanide system.
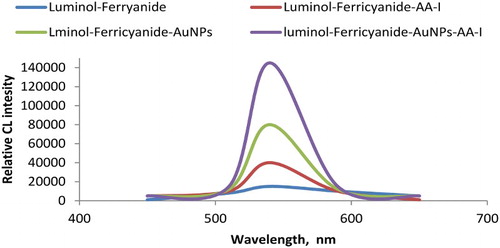
Figure 8. Comparative CL signals using luminol-ferricyanide, luminol-ferricyanide-AA-I, luminol-ferricyanide-AuNPs and luminol-ferricyanide-AuNPs-AA-I: Optimum conditions: 50 µL of 2.0 × 10−4 mol L−1 luminol; 30 µL of AuNPs and 30 µL of 1.0 × 10−2 mol L−1 potassium ferricyanide and 50 µL sample AA-I.