Figures & data
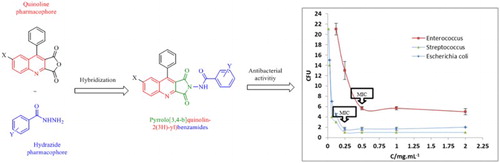
Scheme 1. Synthetic route for the synthesis of pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamide derivatives.
![Scheme 1. Synthetic route for the synthesis of pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamide derivatives.](/cms/asset/9094963a-8502-4c23-819d-e5d58224de4b/tgcl_a_1380233_f0004_b.gif)
Table 1. Synthesis of pyrrolo[3,4-b]quinolin-2(3H)-yl)benzamides 9a–g.
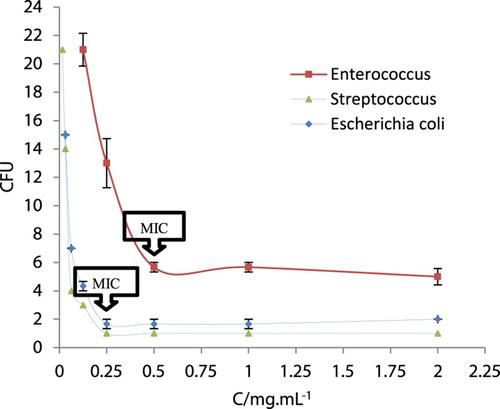
Table 2. Antibacterial activity of 9a–g determined by measuring the diameter of inhibition zone.
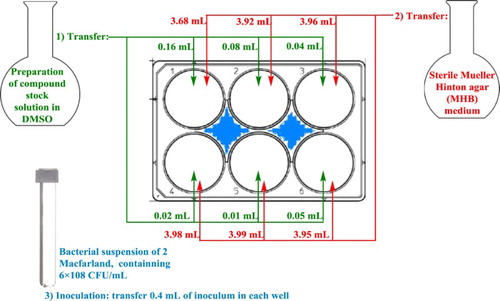
Figure 2. The experimental methodology to perform twofold serial dilution with solid modification. The procedure involves preparing twofold dilutions of the antimicrobial agent (e.g. 0.125, 0.25, 0.5, 1 and 2 mg/mL) in a MHA dispensed in 6-well plate (step 1 and 2). Then, each well is inoculated with a microbial inoculum prepared adjusted to 2 McFarland scale (step 3). After well-mixing, the inoculated 6-well plate are incubated at ∼37°C for 24 h.

![Figure 1. Some important pyrrolo[3,4-b]quinoline-containing drugs.](/cms/asset/9a8bc0c5-6a64-4b68-b7c6-526bc218d54c/tgcl_a_1380233_f0001_b.gif)