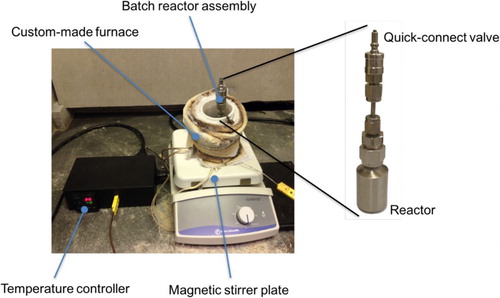
Figures & data
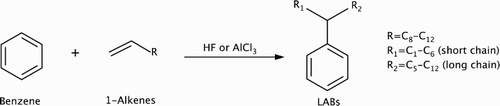
Scheme 1. Current commercial production of linear alkylbenzenes (LABs) from benzene and linear alpha olefins using HF or AlCl3 homogeneous acid catalyst (Citation3).
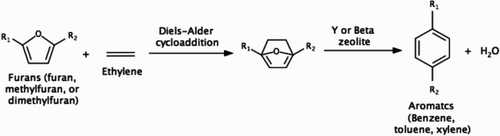
Scheme 2. Production of aromatic hydrocarbons from biomass-derived furans and ethylene (Citation19–28).
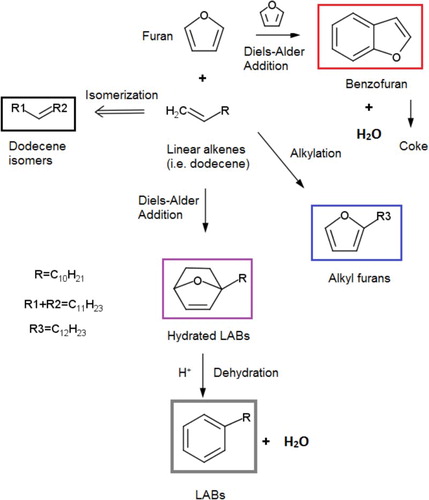
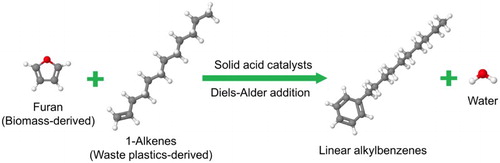
Scheme 3. The new LAB production pathway from furan and linear alpha olefins over solid acid catalysts studied in this work.
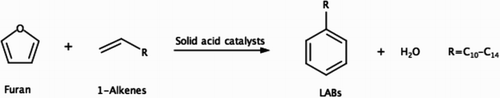
Figure 2. Representative gas chromatograph of reaction mixtures from Diels−Alder reaction between furan and 1-dodecene over niobic acid. The reaction temperature was 250°C, and the reaction time was 6 hours.
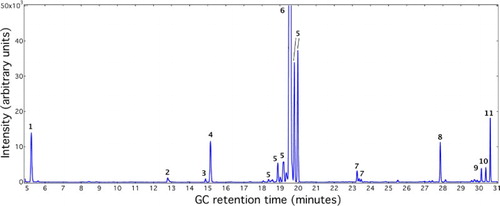
Table 1. Compounds identified by the GC/MS.
Figure 3. The effect of reaction temperature on (A) the conversion of 1-dodecene, (B) the selectivities of major products, and (C) benzofuran to 1-phenyldecane selectivity.
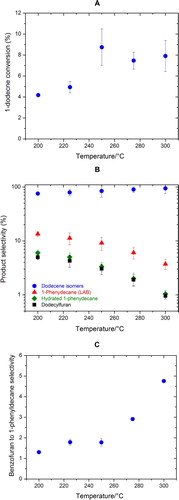
Figure 4. The effect of catalyst loading on (A) the conversion of 1-dodecene, (B) the selectivities of major products, and (C) benzofuran to 1-phenyldecane selectivity.
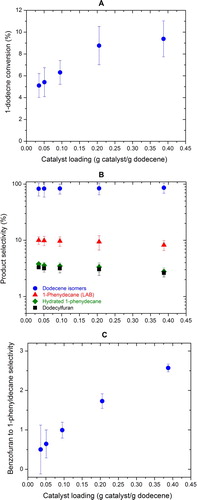
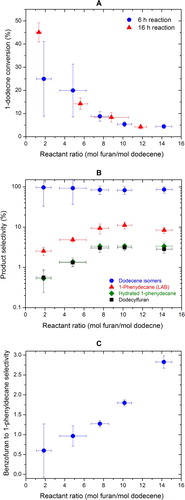
Figure 5. The effect of initial reactant ratio on (A) the conversion of 1-dodecene, (B) the selectivities of major products, and (C) benzofuran to 1-phenyldecane selectivity.