Figures & data
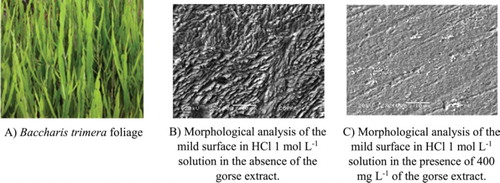
Table 1. Percentage of corrosion inhibition efficiency for some extracts of natural products.
Table 2. Results of gravimetric tests for mild steel by varying the time and concentration of the extract.
Table 3. Results of gravimetric tests in the presence and absence of 200 mg L−1 of the gorse extract for 2 h varying the time.
Figure 1. Arrhenius plots: (A) ln Wcorr × 1/T and (B) ln(Wcorr/T) × 1/T for mild steel in 1 mol L−1 HCl in the absence and presence of gorse extract.
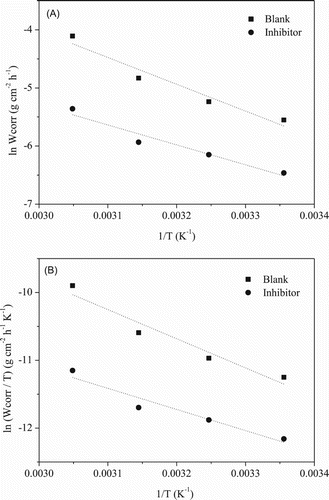
Table 4. Apparent activation energy associated with the corrosion process in the absence and presence of the gorse extract.
Figure 2. Polarization curves for the mild steel in 1 mol L−1 HCl in the absence and presence of different concentrations of gorse extract.
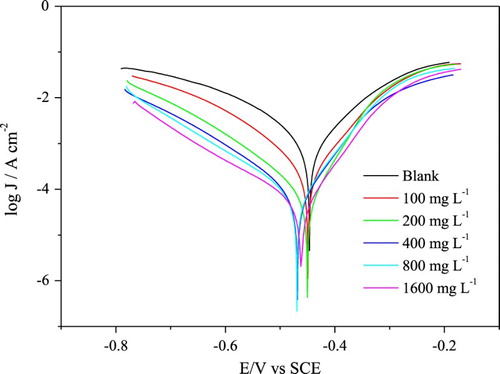
Table 5. Electrochemical parameters obtained from polarization curves.
Figure 3. Electrochemical impedance diagrams for mild steel in 1 mol L−1 HCl medium in the presence and absence of different concentrations of gorse extract.
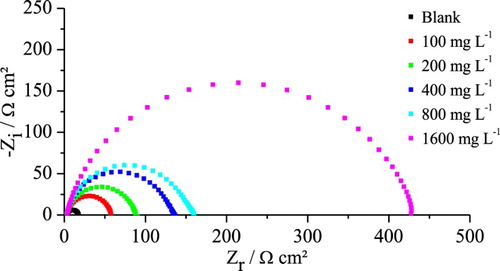
Figure 4. Equivalent circuit used to interpret the electrochemical impedance diagrams obtained for mild steel in the absence and presence of the extract.
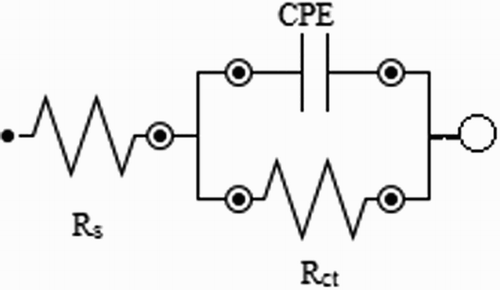
Table 6. Electrochemical parameters obtained from the electrochemical impedance spectroscopy.
Figure 5. Langmuir (A), Temkin (B), Flory–Huggins (C) and El-Awady (D) isotherms for the adsorption of gorse extract on the mild steel surface in 1 mol L−1 HCl solution.
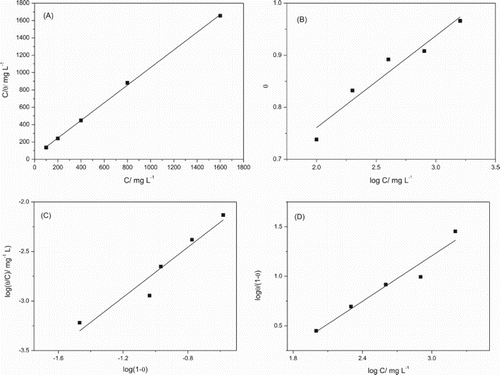
Table 7. Adsorption parameters obtained with gorse extract as corrosion inhibitor for mild steel in 1 mol L−1 HCl.
Table 8. Weight-loss measurements for mild steel in 1 mol L−1 HCl solution in the absence and presence of 5-caffeoylquinic acid (5-CQA), quercetin and HMWF.
Figure 6. Electrochemical impedance diagrams for mild steel in 1 mol L−1 HCl medium in the absence and presence of 100 mg L−1 of HMWF.
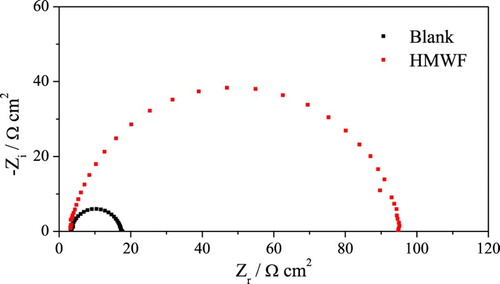
Table 9. Electrochemical parameters obtained from the electrochemical impedance spectroscopy in the presence of 200 mg L−1 of HMWF.
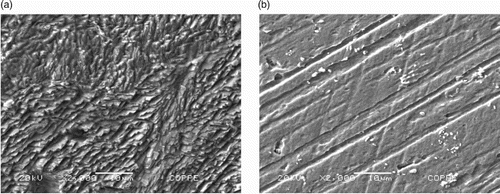
Figure 7. A – Morphological analysis of the mild steel surface in 1 mol L−1 HCl medium in the absence of the gorse extract. B – Morphological analysis of the mild steel surface in 1 mol L−1 HCl medium in the presence of 400 mg L−1 of the gorse extract.