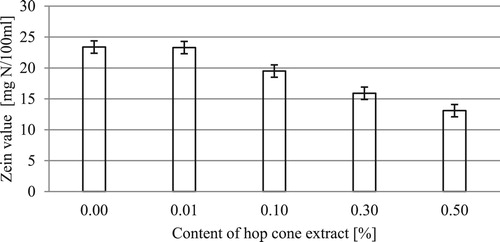
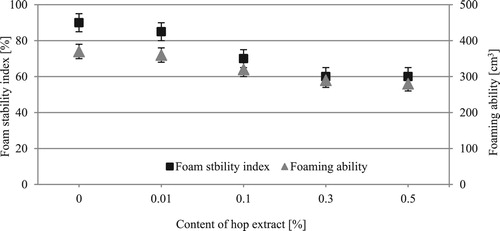
Figures & data
Table 1. Compositions of prototypical formulations.
Table 2. Particle size in the test formulations.
Table 3. Mean values of colorimetric parameters for the test formulations.
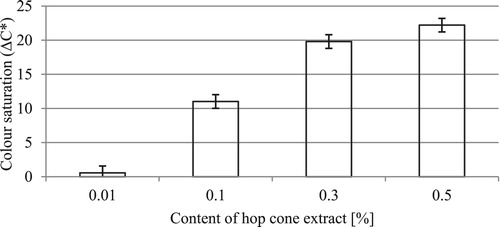
Figure 2. Changes in the total color difference (ΔE*) of the formulations as a function of hop cone extract concentration.