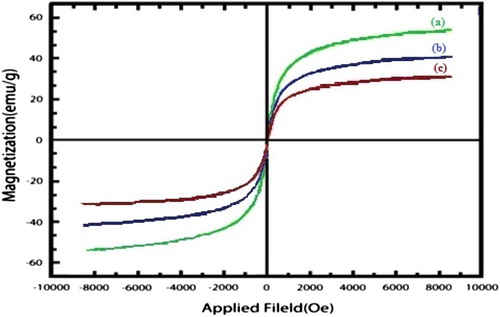
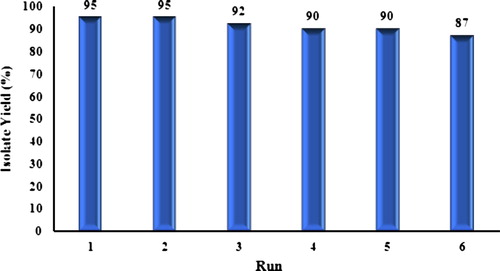
Figures & data
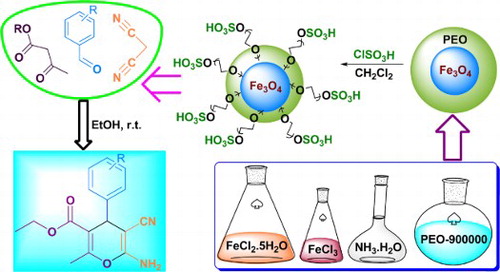
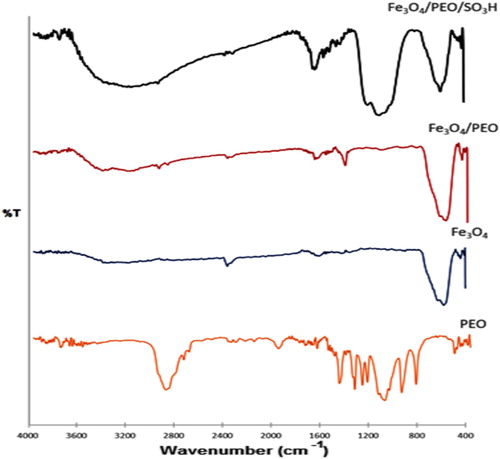
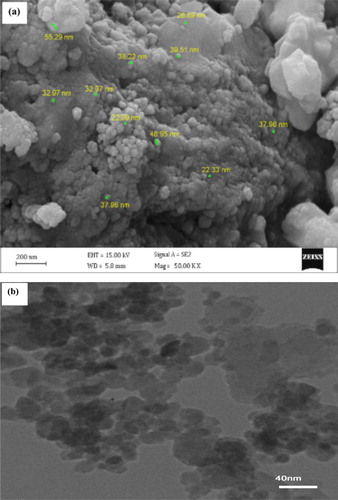
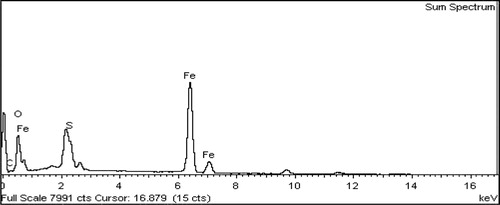
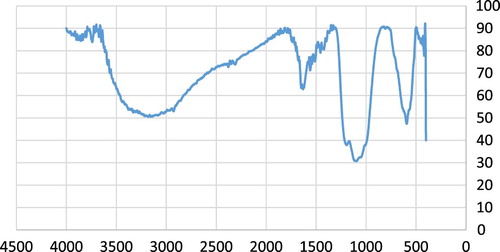
Figure 4. The XRD patterns of Fe3O4@PEO-SO3H composite nanocatalyst. The symbol “*” represents the PEO-SO3H peak.
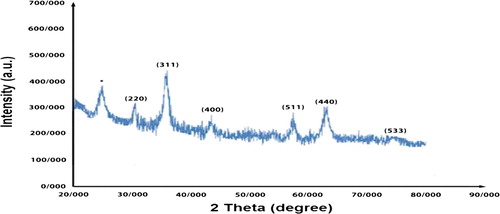
Table 1. Optimizing of the reaction conditions in the synthesis of 4b.
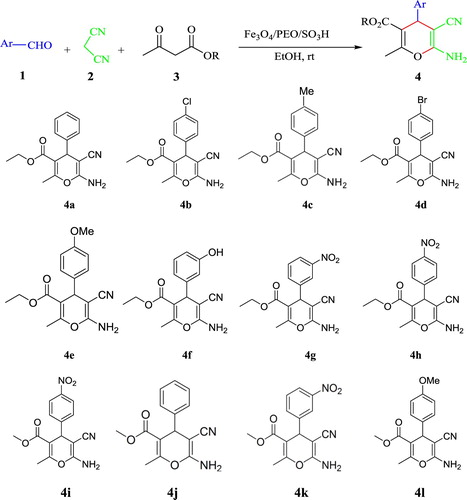
Table 2. Synthesis of 2-amino-4H-pyrans derivatives using Fe3O4@PEO-SO3H nanocatalyst.
Table 3. Comparisons of catalysts and its components effects on the model reaction.
Supplemental material