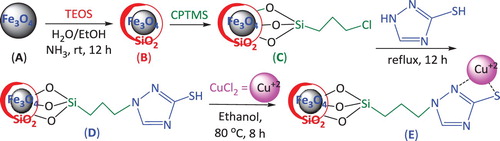
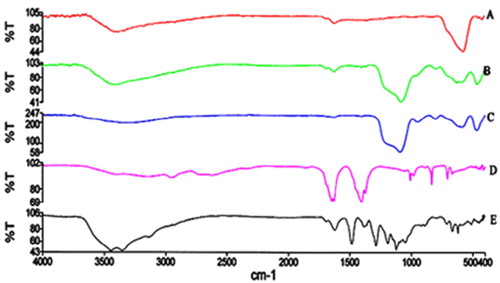
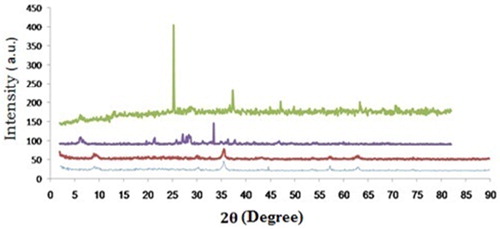
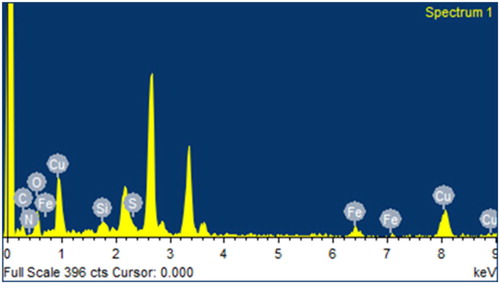
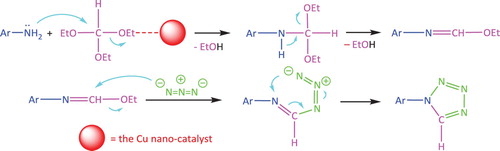
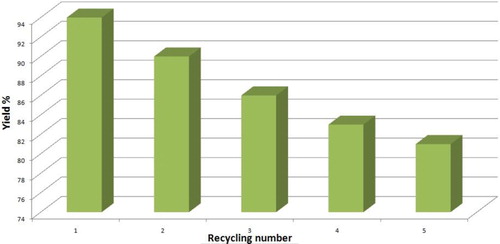
Figures & data
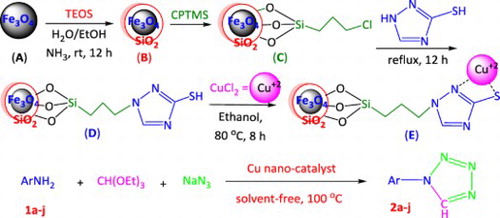
Table 1. Effect of the amount of the catalyst on the synthesis of 1-(4-nitrophenyl)-1H-tetrazole in solvent-free condition at 100°C.
Table 2. Effect of solvent on the synthesis of 1-(4-nitrophenyl)-1H-tetrazole at 100°C.
Table 3. Effect of temperature on the synthesis of 1-(4-nitrophenyl)-1H-tetrazole in solvent-free condition.
Table 4. Synthesis of various tetrazoles (2a–j).
Supplemental material