Figures & data
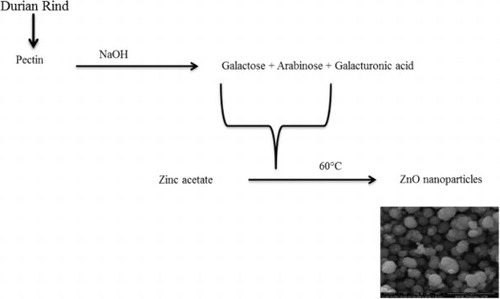
Figure 1. Proposed mechanism for durian rind aqueous extract mediated formation of zinc oxide nanoparticles.
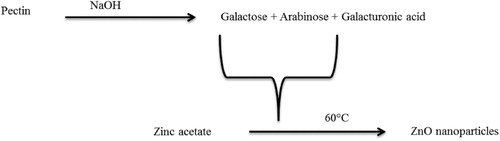
Figure 4. (A) SEM image of ZnO NPs at 400 nm resolution, (B) TEM image of ZnO NPs, (C) XRD pattern of ZnO NPs, (D) EDX spectra of ZnO NPs.
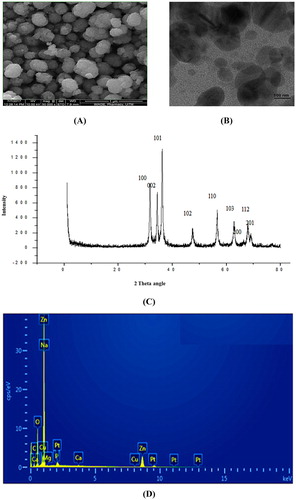
Figure 5. (A) Size distribution of ZnO NPs obtained from dynamic light scattering, (B) Zeta potential of ZnO NPs.
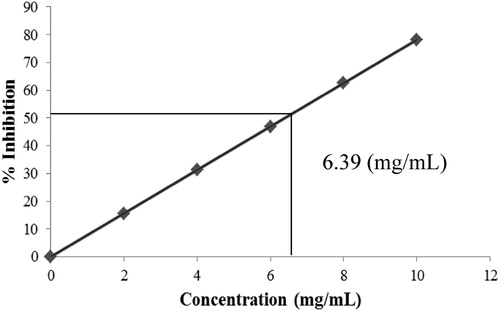
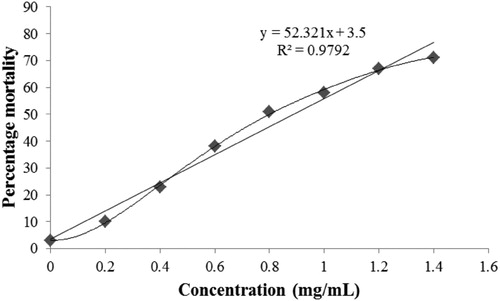
Table 1. Antimicrobial activities of ZnO NPs.
Figure 7. Degradation of methylene blue under solar irradiation: (A) in absence of ZnO NPs at pH 10, (B) in the presence of ZnO NPs at pH 10.
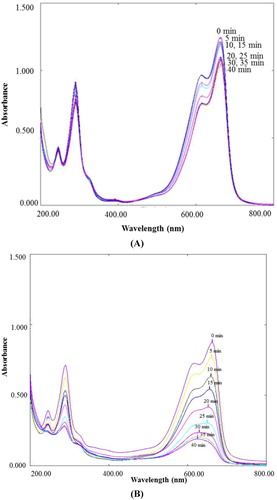
Figure 8. (A) Photocatalytic degradation efficiency of ZnO NPs, (B) Percentage degradation of methylene blue in the presence and absence of ZnO NPs under solar irradiation.
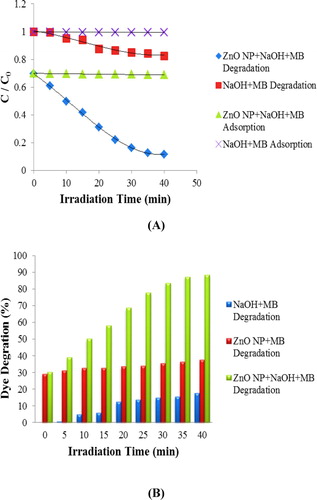
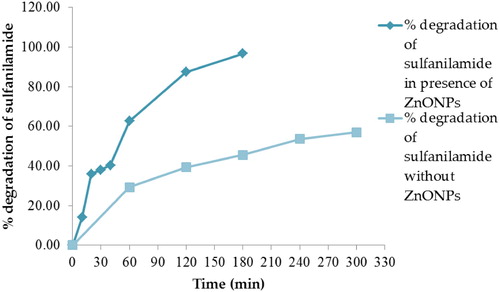
Figure 10. Percentage degradation of sulfanilamide at pH 7, 9, 10, 11and 12 in different time interval under natural sunlight.
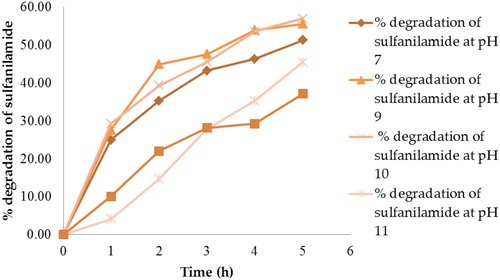
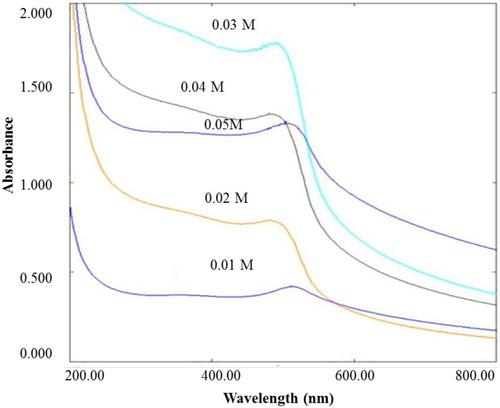
Figure 11. UV-Vis spectra of degradation of sulfanilamide at different time interval in the presence of 0.10% ZnO NPs under natural sunlight.
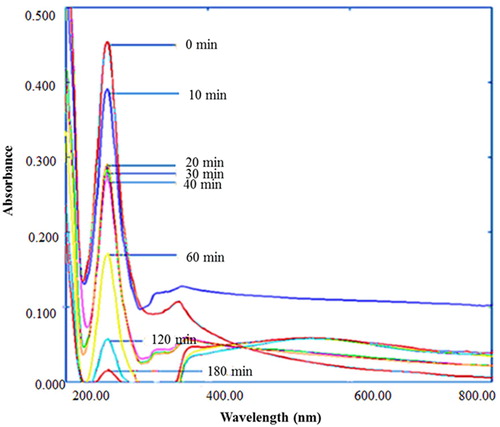
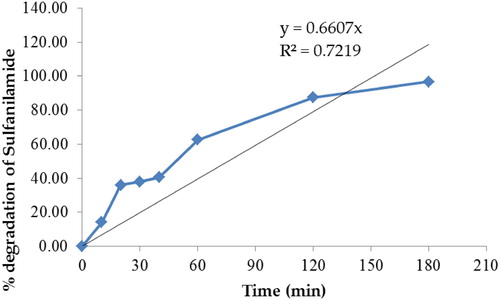
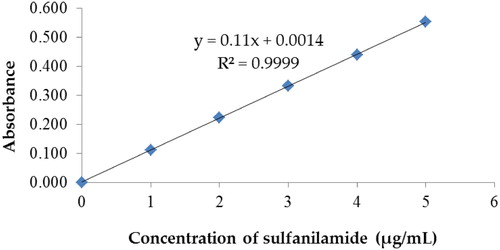
Figure 12. Percentage degradation of sulfanilamide at different time interval in the presence of 0.10% ZnO NPs under natural sunlight.