Figures & data
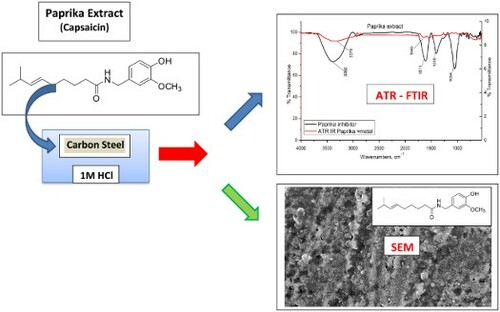
Table 1. Variation of the corrosion rate, and inhibition efficiency, with the concentration of the Paprika extract at different temperatures.
Figure 2. Langmuir adsorption isotherms for the Paprika extract on carbon steel different temperatures.
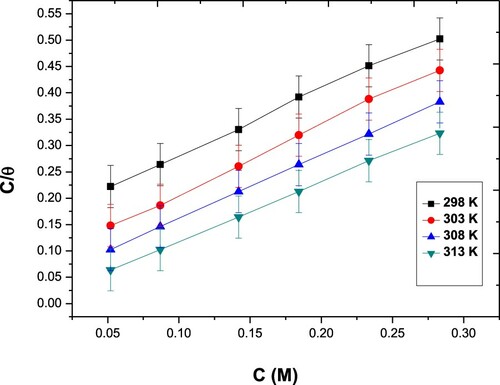
Figure 3. Frumkin adsorption isotherms for the Paprika extract on carbon steel different temperatures.
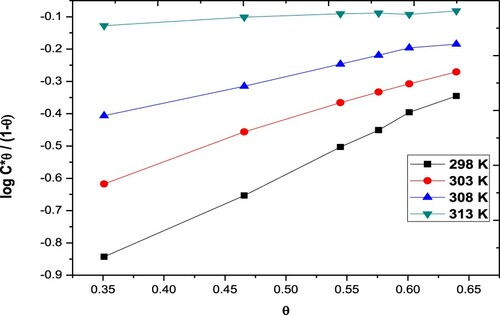
Figure 4. Freundlich adsorption isotherms for the Paprika extract on carbon steel different temperatures.
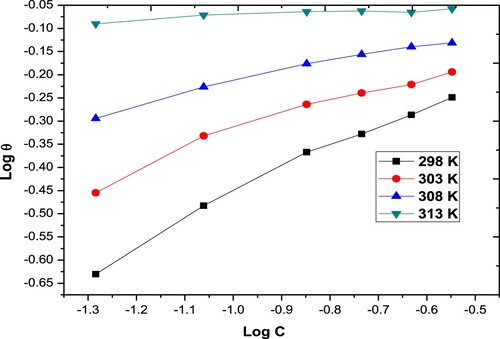
Figure 5. Temkin adsorption isotherms for the Paprika extract on carbon steel different temperatures.
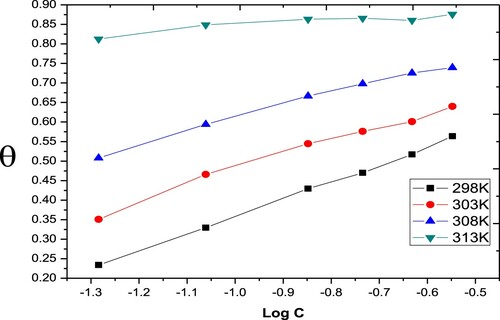
Table 2. Adsorption parameters of the Paprika extract on carbon steel in 1M HCl at different temperatures.
Figure 6. Potentiodynamic Polarization curves for carbon steel in 1 M HCl without and with various concentrations of the Paprika extract at 298 K.
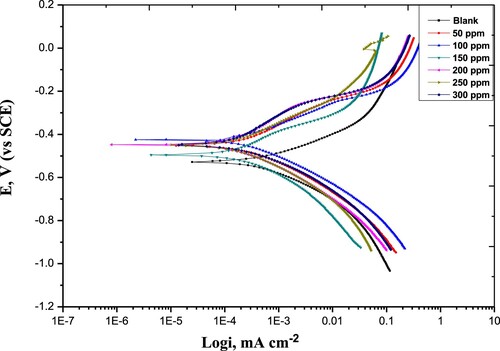
Table 3. Potentiodynamic polarization parameters of carbon steel in 1M HCl without and with various concentrations of the investigated extract at 25°C.
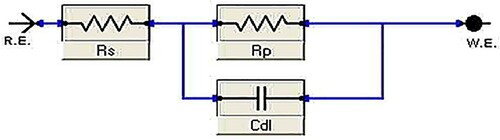
Figure 7. The Nyquist (a) and Bode-phase angle (b) plots of carbon steel in 1 M HCl in the absence and presence of various concentrations of the Paprika extract at 298 K.
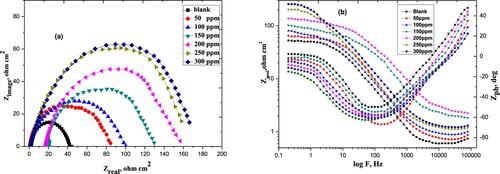
Table 4. EIS parameters for carbon steel corrosion in the absence and presence of various concentrations of the Paprika extract at 25°C.
Figure 9. EFM data for carbon steel in 1 M HCl in the absence and presence of different concentrations of the Paprika extract at 298 K.
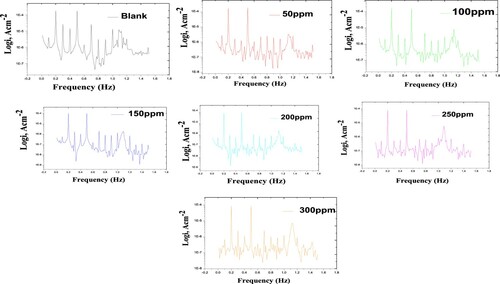
Table 5. EFM parameters for carbon steel in 1 M HCl in the absence and presence of different concentrations of the Paprika extract.
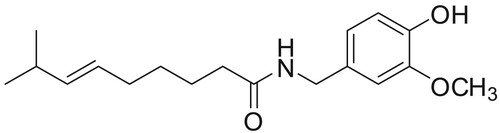
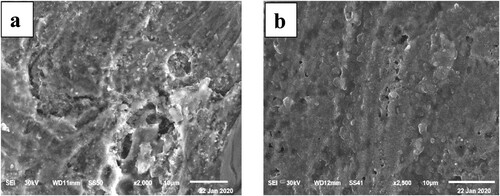
Figure 10. ATR-IR spectra of the Paprika extract and Paprika extract in the presence of 1M HCl, and carbon steel.
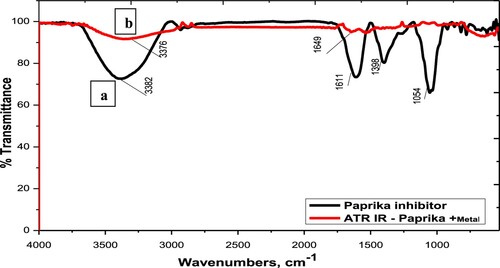
Table 6. Comparison between the tested Paprika extract and other plant extracts reported in the literature.