Figures & data
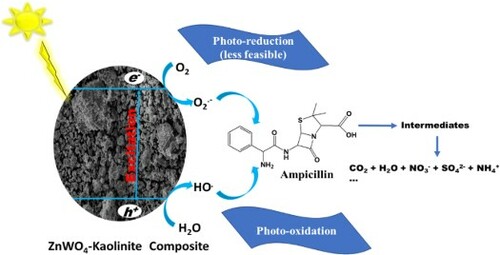
Figure 1. (A) X-ray diffractogram (XRD) and (B) Fourier transform infrared (FTIR) spectra of kaolinite, Zn(CH3CO2)2/kaolinite (ZnK), Na2WO4/Kaolinite (WK), Zn(CH3CO2)2/Na2WO4-Kaolinite calcined at 300°C (ZnWK-3), (E) Zn(CH3CO2)2/Na2WO4/Kaolinite calcined at 500°C (ZnWK-5) and Zn(CH3CO2)2/Na2WO4/Kaolinite calcined at 700°C (ZnWK-7) [K = kaolinite, Q = quartz, S = sanmartinite (ZnWO4) and Z = ZnO].
![Figure 1. (A) X-ray diffractogram (XRD) and (B) Fourier transform infrared (FTIR) spectra of kaolinite, Zn(CH3CO2)2/kaolinite (ZnK), Na2WO4/Kaolinite (WK), Zn(CH3CO2)2/Na2WO4-Kaolinite calcined at 300°C (ZnWK-3), (E) Zn(CH3CO2)2/Na2WO4/Kaolinite calcined at 500°C (ZnWK-5) and Zn(CH3CO2)2/Na2WO4/Kaolinite calcined at 700°C (ZnWK-7) [K = kaolinite, Q = quartz, S = sanmartinite (ZnWO4) and Z = ZnO].](/cms/asset/45232474-39ba-4e5a-9803-500e6492211d/tgcl_a_2124889_f0001_ob.jpg)
Figure 2. Scanning electron micrograph (SEM) of (A) kaolinite, (B) Zn(CH3CO2)2/kaolinite (ZnK), (C) Na2WO4/Kaolinite (WK), (D) Zn(CH3CO2)2/Na2WO4-Kaolinite calcined at 300°C (ZnWK-3), (E) Zn(CH3CO2)2/Na2WO4/Kaolinite calcined at 500°C (ZnWK-5) and (F) Zn(CH3CO2)2/Na2WO4/Kaolinite calcined at 700°C (ZnWK-7).

Table 1. Energy-dispersive X-ray (EDX) analysis for spectra of kaolinite, Zn(CH3CO2)2/kaolinite (ZnK), Na2WO4/Kaolinite (WK), Zn(CH3CO2)2/Na2WO4-Kaolinite calcined at 300°C (ZnWK-3), Zn(CH3CO2)2/Na2WO4/Kaolinite calcined at 500°C (ZnWK-5) and Zn(CH3CO2)2/Na2WO4/Kaolinite calcined at 700°C (ZnWK-7).
Figure 3. (A) Absorption spectra, (B) Tauc plots and (C) photoluminescence spectra (at the excitation wavelength of 380 nm) of kaolinite, Zn(CH3CO2)2/kaolinite (ZnK), Na2WO4/Kaolinite (WK), Zn(CH3CO2)2/Na2WO4-Kaolinite calcined at 300°C (ZnWK-3), Zn(CH3CO2)2/Na2WO4/Kaolinite calcined at 500°C (ZnWK-5) and Zn(CH3CO2)2/Na2WO4/Kaolinite calcined at 700°C (ZnWK-7).
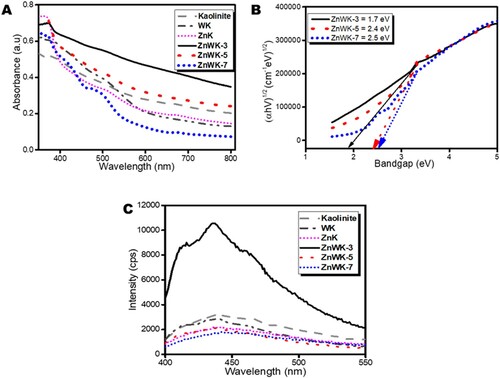
Figure 4. X-band Electron Paramagnetic Resonance spectra of Zn(CH3CO2)2/Na2WO4/Kaolinite photocatalytic composite (ZnWK-5) calcined at 500°C.
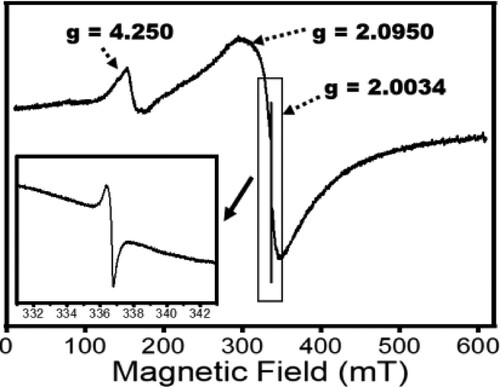
Figure 5. X-ray photoelectron spectroscopy (XPS) (A) scan, (B) Zn 2p spectrum, (C) W 4f spectrum, (D) O 1s spectrum and (E) C 1s spectrum of Zn(CH3CO2)2/Na2WO4/Kaolinite photocatalytic composite (ZnWK-5) calcined at 500°C.
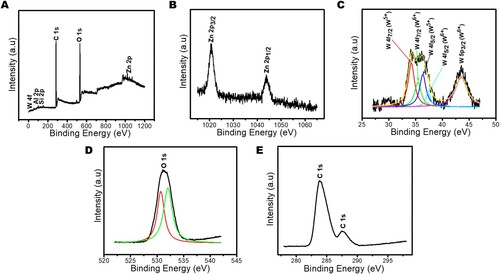
Figure 6. Photocatalysts screening with 50 mL of 50 mg/L Ampicillin under dark and solar irradiation for 120 min using 50 mg/L of kaolinite and the prepared composites.
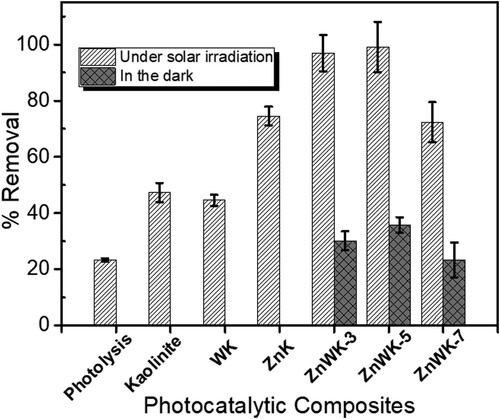
Figure 7. (A) Time profile plot showing the removal of Ampicillin from water, (B) Langmuir-Hinshelwood (L-H) kinetic model plot for the removal of AMP in water, (C) mineralization of AMP in water in terms of TOC removal, and (D) Inorganic by-products (anions) from mineralization of AMP in water.
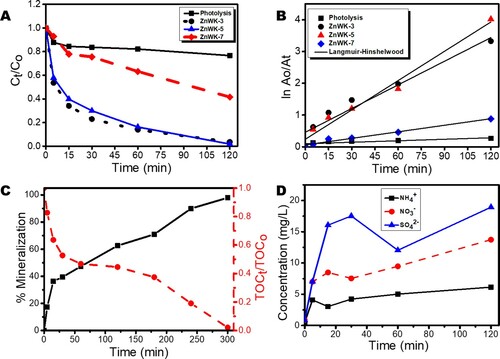
Table 2. Kinetic parameters for the photodegradation of ampicillin (AMP).
Scheme 1. Degradation products for the degradation of ampicillin using Zn(CH3CO2)2/Na2WO4/Kaolinite photocatalytic composite (ZnWK-5) calcined at 500°C.
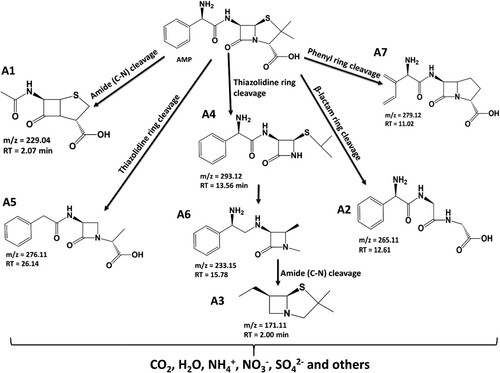
Figure 8. Result of toxicity test of (A) Escherichia coli DSM 10974 and (B) Staphylococcus aureus ATCC 25923 contaminated water before and after treatment with ZnWK-5 composite
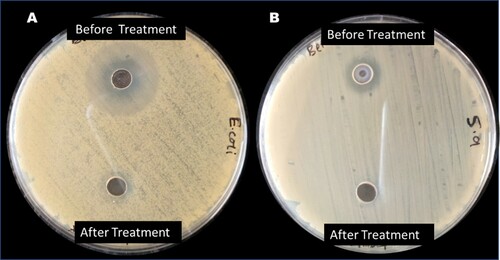
Figure 9. Plots of the effect of change in (A) Photocatalyst dose at fixed AMP concentration, (B) initial AMP concentration at fixed catalyst dose, (C) AMP solution pH (D) Ionic strength of the AMP solution, on the photodegradation of 50 mg/L of the AMP in water.
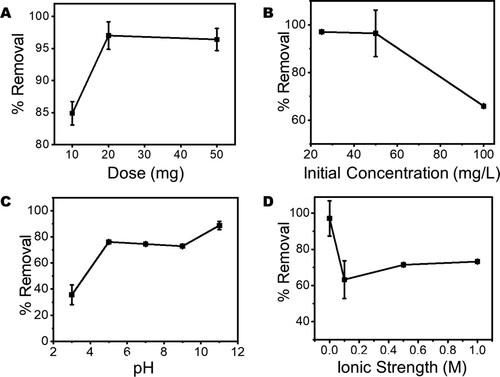
Figure 10. The effect of (A) the presence of inorganic anions and (B) radical scavengers on the photodegradation efficiency of Ampicillin (AMP) molecules in water using ZnWK-5 composite (IPA = isopropyl alcohol, BZQ = benzoquinone and NaOX = sodium oxalate).
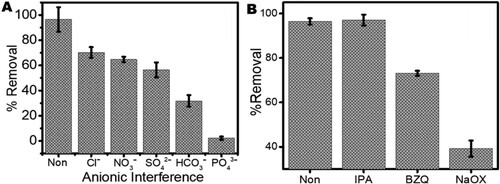
Scheme 2. Photodegradation scheme for the degradation of ampicillin in water by Zn(CH3CO2)2/Na2WO4/Kaolinite photocatalytic composite (ZnWK-5) calcined at 500°C.
Figure 11. Stability of the efficiency of ZnWK-5 composite over five reuse cycles for the photodegradation of ampicillin (AMP) in water.
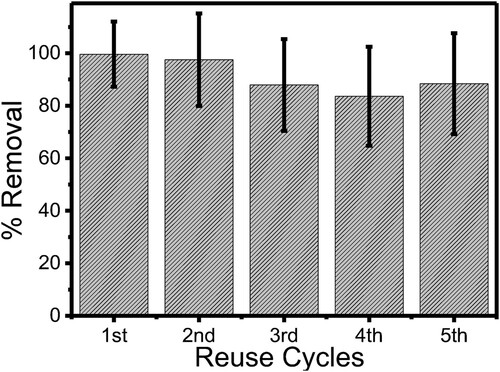
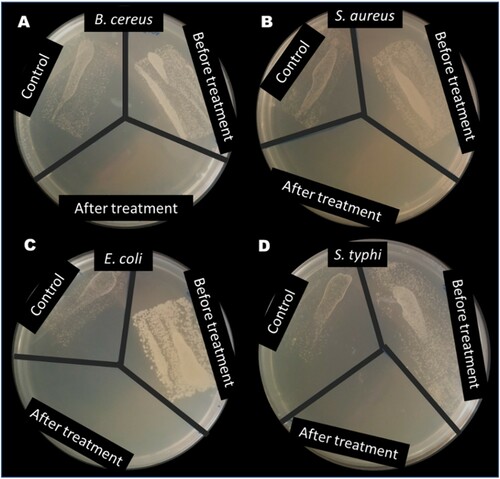
Figure 12. Test result from disinfection of water containing (A) B. Cereus, (B) S. aureus, (C) E. coli and (D) S. typhi using ZnWK-5 photocatalytic composite.