Figures & data
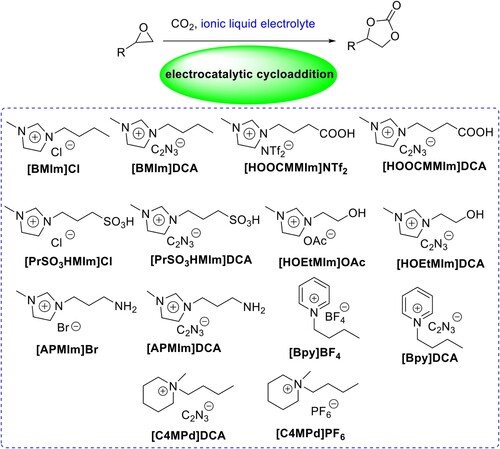
Table 1. Screening of supporting ionic liquid electrolytes for the cycloaddition of propylene oxide with CO2 to produce propylene carbonate.Table Footnotea
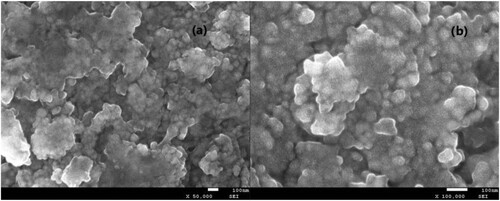
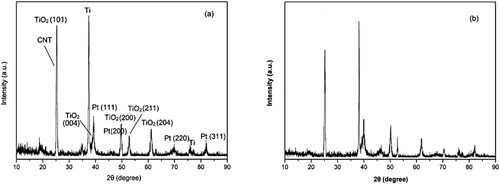
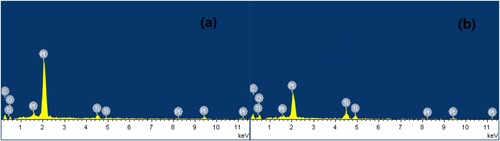
Figure 1. Effect of reaction parameters on the electrocatalytic cycloaddition (a) Effect of electrode material on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, current density 3.4 mA cm−2, 50°C, 2 h), (b) effect of current density on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, 50°C, 2 h), (c) effect of reaction temperature on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, current density 3.4 mA cm−2, 2 h), and (d) effect of reaction time on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, current density 3.4 mA cm−2, 50°C).
![Figure 1. Effect of reaction parameters on the electrocatalytic cycloaddition (a) Effect of electrode material on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, current density 3.4 mA cm−2, 50°C, 2 h), (b) effect of current density on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, 50°C, 2 h), (c) effect of reaction temperature on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, current density 3.4 mA cm−2, 2 h), and (d) effect of reaction time on the cycloaddition (10 mmol propylene oxide, balloon CO2, 15 mL acetonitrile, 10 mL [APMIm]DCA, graphite anode, Ti/TiO2-CNT-Pt cathode, current density 3.4 mA cm−2, 50°C).](/cms/asset/cd9dcd94-3e0b-46b7-ab96-8f973a0e9853/tgcl_a_2163192_f0001_oc.jpg)
Figure 2. Recycling stability of the electro-catalytic system (propylene oxide (10 mmol), CO2 (balloon), acetonitrile (15 mL), recovered supporting ionic liquid electrolyte [APMIm]DCA, used graphite anode and Ti/TiO2-CNT-Pt cathode in a single compartment cell with current density 3.4 mA cm−2 at 50°C for 2 h).
![Figure 2. Recycling stability of the electro-catalytic system (propylene oxide (10 mmol), CO2 (balloon), acetonitrile (15 mL), recovered supporting ionic liquid electrolyte [APMIm]DCA, used graphite anode and Ti/TiO2-CNT-Pt cathode in a single compartment cell with current density 3.4 mA cm−2 at 50°C for 2 h).](/cms/asset/a349372e-0692-4c32-aed2-89b21ff3dc14/tgcl_a_2163192_f0002_oc.jpg)
Table 2. Electro-catalytic synthesis of cyclic carbonates from epoxides and CO2.Table Footnotea