Figures & data
Table 1. The nature of base catalysts influenced the product formation in ‘one-pot’ Baker–Venkataraman rearrangement.Table Footnotea
Scheme 2. Sequential ‘one-pot’ synthesis of 3-benzoyl-6-chloroflavone via Baker–Venkataraman rearrangement.
Figure 1. Influence of choline hydroxide amount in the synthesis of 3-benzoyl-6-chloroflavone from the reaction of 5′-chloro-2′-hydroxyacetophenone (1.0 mmol) and benzoyl chloride (2.3 mmol). Reaction conditions: step 1: Et3N (2.5 mmol) under r.t. stirring for 30 min., and step 2: ChOH (varied amount) in Et3N (11.0 mmol) under reflux for 5 hours.
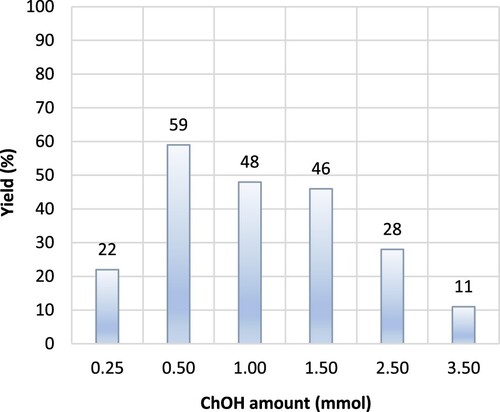
Table 2. Yields of 3-aroylflavones from the reactions between 2′-hydroxyacetophenones and benzoyl chlorides.Table Footnotea
Scheme 3. A plausible mechanism for the synthesis of 3-aroylflavones via sequential ‘one-pot’ Baker – Venkataraman rearrangement catalyzed by choline hydroxide in triethylamine.
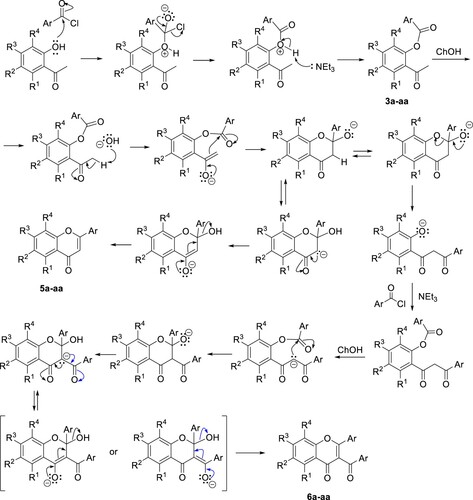
Figure 2. Reusability of choline hydroxide in sequential ‘one-pot’ synthesis of 3-benzoyl-6-chloroflavone. Yields were calculated based on HPLC-UV analyses.
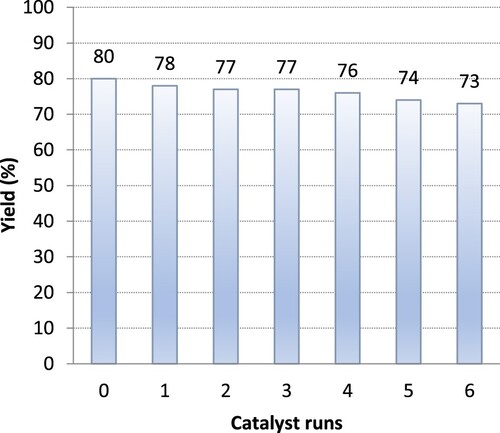
Figure 3. FT–IR spectra of fresh ChOH (a), ChOH after 1st recycle (b), and ChOH after 6th recycle (c).
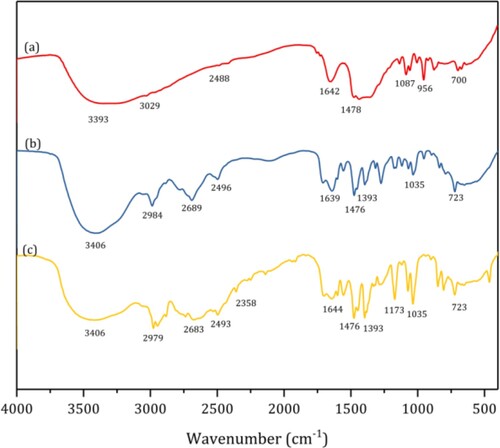
Figure 4. FT–IR spectra of pairs of 6,8-dichloroflavone (Fl-3,5Cl) and 6,8,3′-trichloro-3-(3″-chlorobenzoyl)flavone (BzFl-3,5Cl) (a), pairs of 5-hydroxyflavone (Fl-6OH) and 3-benzoyl-5-hydroxyflavone (BzFl-6OH) (b), six 3-benzoylflavones (c), and five 3-aroylflavones (d).
Table 3. Comparison of previous synthesis of 3-aroylflavones from 2′-hydroxyacetophenones and benzoyl chlorides.