Figures & data
Figure 3. Effect of the CeO2 NPs concentration on the OCP value for LDX 2101 duplex stainless steel in a CO2-saturated 3.5% NaCl solution.
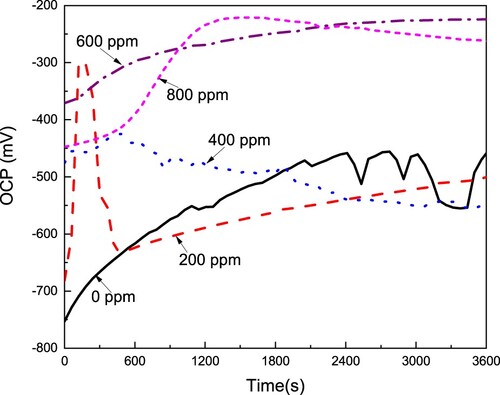
Figure 4. Effect of the CeO2 NPs concentration on the polarization curves for LDX 2101 duplex stainless steel in a CO2-saturated 3.5% NaCl solution.
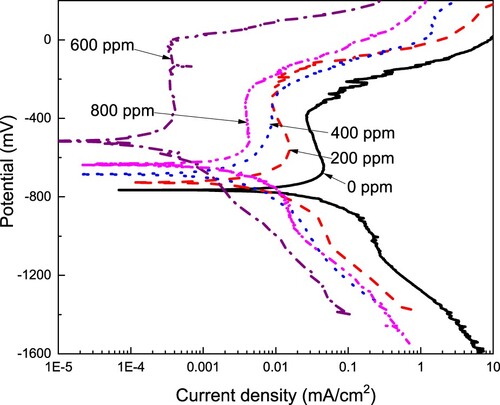
Table 1. Electrochemical parameters obtained from polarization curves.
Figure 5. Langmuir adsorption isotherm for LDX 2101 duplex stainless steel in a CO2-saturated 3.5% NaCl solution containing CeO2 NPs.
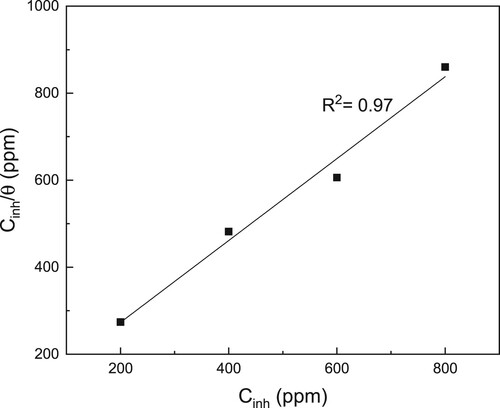
Figure 6. Effect of the CeO2 NPs concentration on the Rp value for LDX 2101 duplex stainless steel in a CO2-saturated 3.5% NaCl solution.
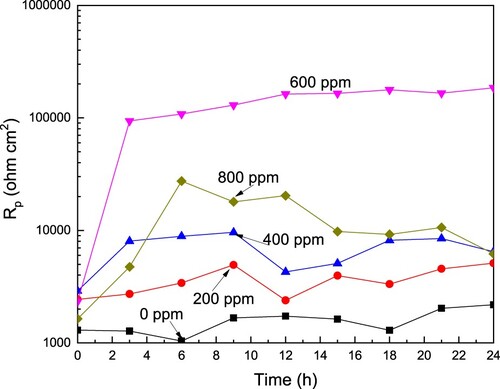
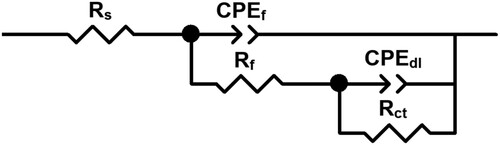
Figure 7. (a) Nyquist and (b) Bode diagrams for LDX 2101 duplex stainless steel in uninhibited CO2–saturated 3.5% NaCl solution.
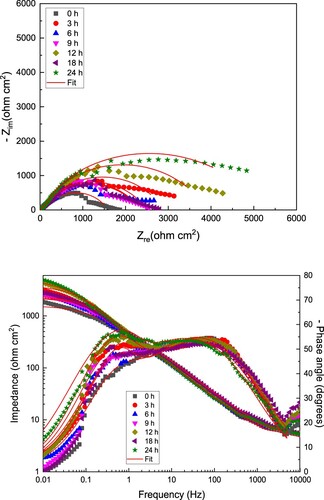
Figure 8. (a) Nyquist and (b) Bode diagrams for LDX 2101 duplex stainless steel in CO2-saturated 3.5% NaCl solution containing 600 ppm of CeO2 NPs.
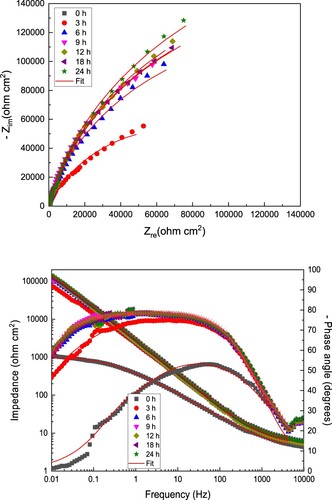
Table 2. Electrochemical parameters obtained from fitting the EIS data for uninhibited solution.
Table 3. Electrochemical parameters obtained from fitting the EIS data for tests containing 600 ppm of CeO2 NPs.
Data availability statement
The raw/processed data required to reproduce these findings are available upon request.