Figures & data
Table 1. Summary of the reported review previous studies compared with the present study.
Table 2. The chemical percent composition of the studied metal alloy.
Figure 1. Scheme 1 shows the chemical green synthesis route for the amide-based nonionic polymeric surfactant (PETTEDAA-Oli) starting from PET waste.
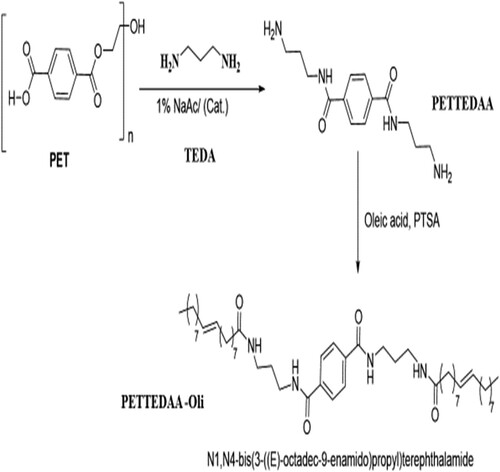
Figure 2. Scheme 2 shows the chemical green synthesis route for the glycol-based nonionic polymeric surfactant (PETTEG-Oli) starting from PET waste.
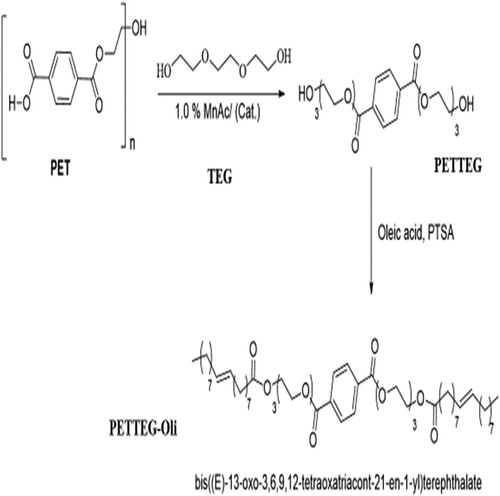
Table 3. Show the elemental analysis of the prepared surfactants materials from PET.
Figure 3. Depicts the weight loss of steel in 3.5% NaCl over time with and without the presence of a prepared polymeric surfactant (PETTEDAA -Oli) inhibitor derived from plastic waste.
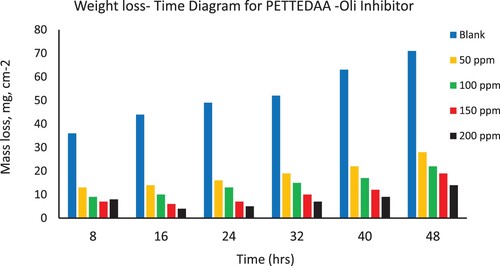
Figure 4. Depicts the weight loss of steel in 3.5% NaCl over time with and without the presence of a polymeric surfactant (PETTEDAA) inhibitor derived from plastic waste.
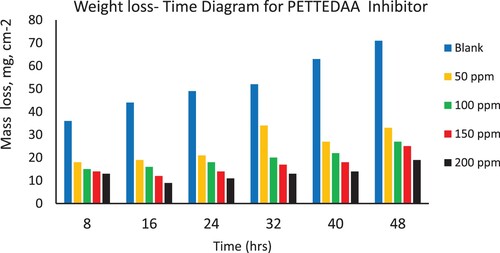
Figure 5. Depicts the weight loss of steel in 3.5% NaCl over time with and without the presence of a polymeric surfactant (PETTEG-Oli) inhibitor derived from plastic waste.
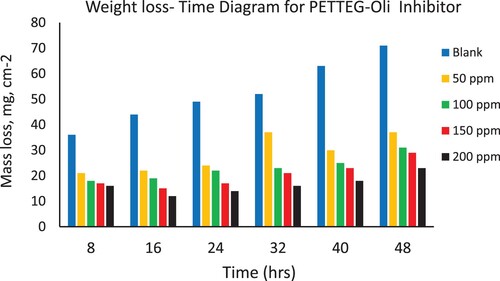
Figure 6. Depicts the weight loss of steel in 3.5% NaCl over time with and without the presence of a prepared polymeric surfactant (PETTEG) inhibitor derived from plastic waste.
Table 4. Corrosion inhibition parameters for various concentrations of the prepared nonionic surfactants at 303 k, for c-steel in marine environment data from Mass loss.
Figure 7. Temperature inhibition relationship from mass loss data for 200 ppm of the four studied surfactants derived from PET waste used as inhibitor for steel in marine environment.
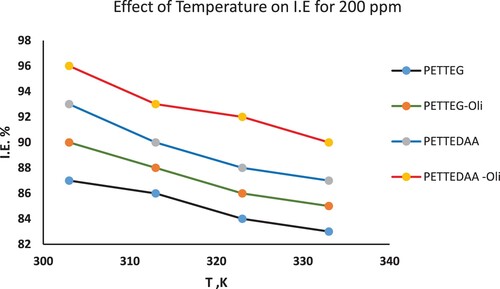
Figure 8. Graphical presentation of the Atomic Absorption Spectroscopy (AAS) data of the prepared surfactants from PET waste used as inhibitor for steel in 3.5% NaCl solution.
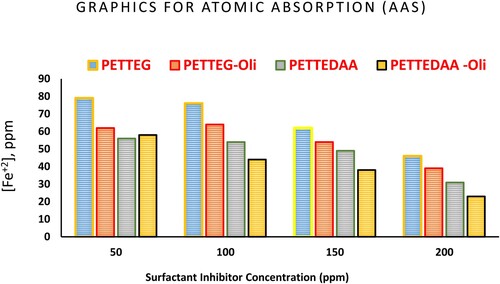
Table 5. Atomic absorption spectroscopy results from corrosion of steel alloy in marine environment in the presence and absence of the prepared polymeric surfactants.
Table 6. Inhibited artificial NaCl solution thermometric parameters by the prepared polymeric surfactants as inhibitor for c-steel.
Figure 9. Adsorption of the four prepared surfactant inhibitors on the C-steel surface immersed in 3.5% NaCl solution at 30 °C according Langmuir adsorption isotherm model.
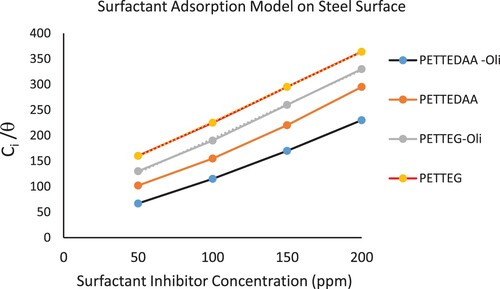
Figure 10. Open Circuit Potential (OCP) vs time measurements for C- steel immersed in 3.5% NaCl solution in the absence and presence of nonionic surfactant inhibitor (PETTEDAA -Oli) compound derived from recycled PET waste.
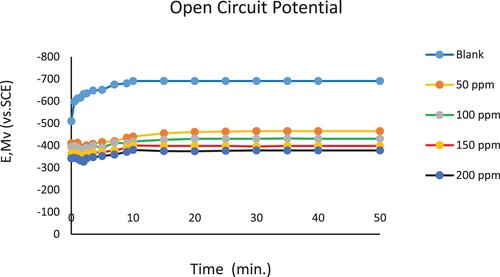
Figure 11. Tafel curves (Potentiodynamic Polarization) for C-steel immersed in .5% NaCl solution at 30 °C in the absence and presence of nonionic surfactant inhibitor (PETTEDAA -Oli) compound derived from recycled PET waste.
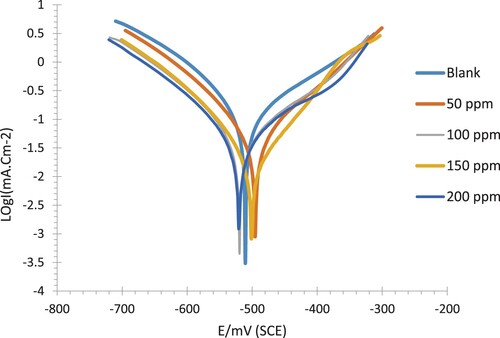
Table 7. Potentiodynamic (PDP) polarization parameters for corrosion and inhibition of steel by the surfactant polymer compound (PETTEDAA -Oli) in 3.5% NaCl solution at 303 k.
Figure 12. Nyquist plots for a carbon steel electrode in 3.5% NaCl solution at 30 °C with various concentrations of the prepared nonionic surfactant (PETTEDAA -Oli) inhibitor derived from plastic waste.
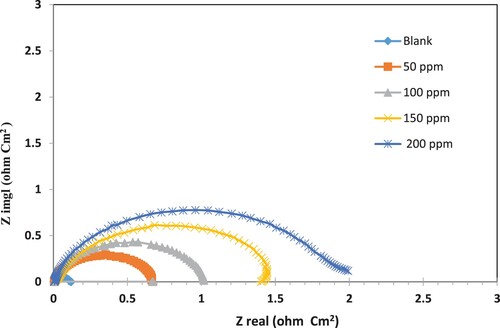
Table 8. Shows the electrochemical impedance spectroscopy (EIS) parameters of steel corrosion by3.5% NaCl at 303 k in the presence and absence of different concentrations from the prepared surfactant inhibitor (PETTEDAA -Oli).
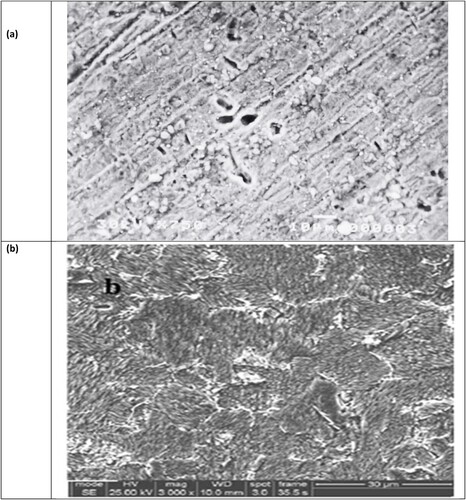
Figure 13. SEM images of carbon steel sample immersion in 3.5% NaCl solution for 48 hr. at 30 °C: (a) with the presence of 200 ppm of the inhibitor (PETTEDAA -Oli)). And (b) in absence of inhibitor.