Figures & data
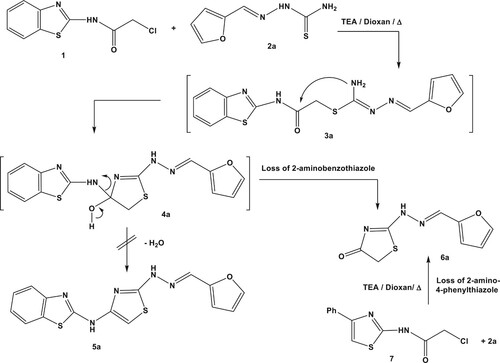
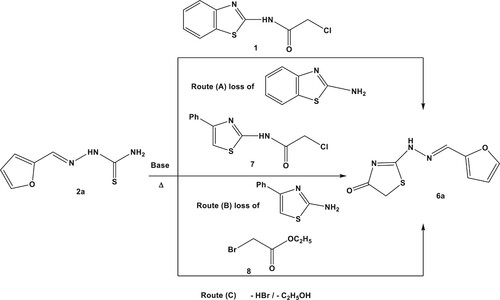
Table 1. A comparative yield percentage of compound 6a via different synthetic pathways.
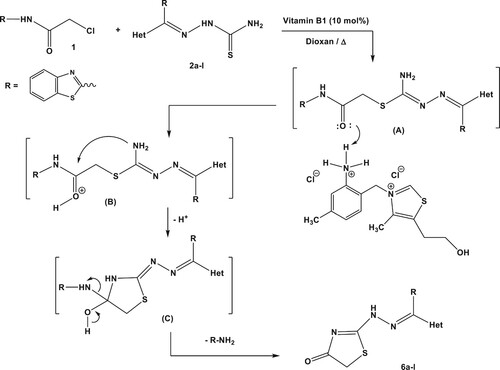
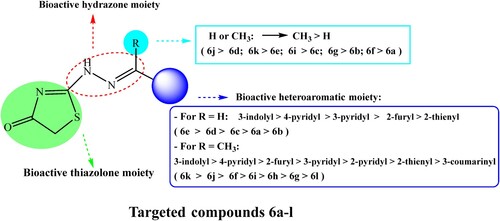
Table 2. Percentage yields of 2-hydrazonothiazolidin-4(5H)-ones (6a-m).
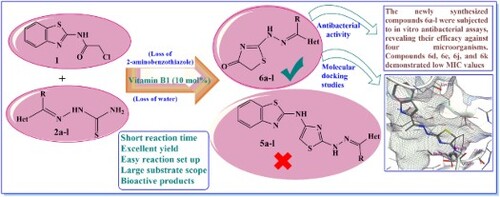
Table 3. Preliminary antibacterial activity for compounds 6a-l (10 mM).
Table 4. MICs of the synthesized compounds against the tested pathogenic bacteria.Table Footnotea
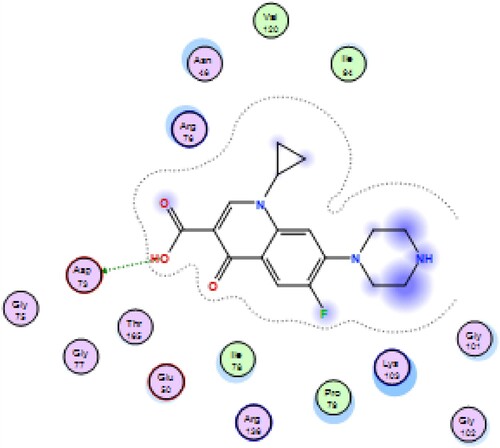
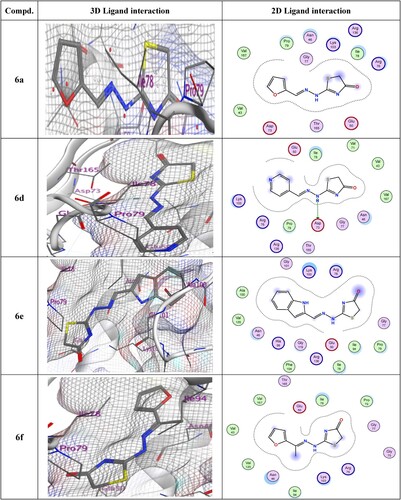
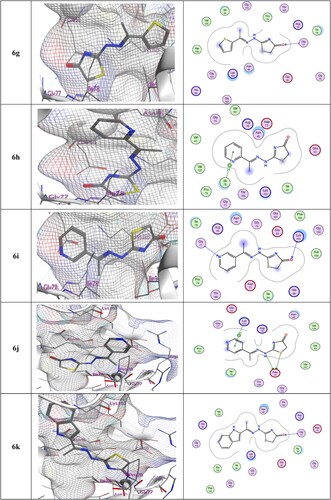
Table 5. Docking score (kcal/mol), No. of H-bonding, No. of arene interaction, and RMSD of most potent synthesized compounds with 4DUH receptor when compared to Ciprofloxacin.
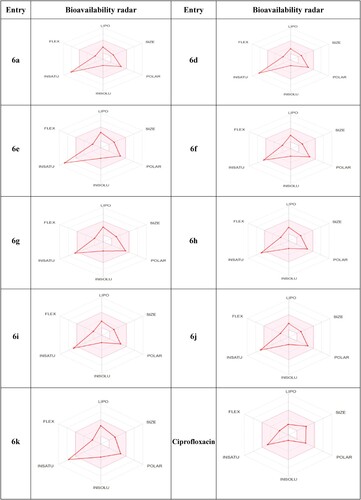
Table 6. The physicochemical properties, lipophilicity, pharmacokinetic characteristics, and drug likeness matching of the most potent thiazolidinone derivatives.