Figures & data
Table 1. Selected reviews on Quaternary floristic and vegetational history of different geographical areas. See Birks HJB and Berglund (Citation2018) for further examples.
Table 2. Selected reviews of contributions of Quaternary botany to modern ecology or biogeography.
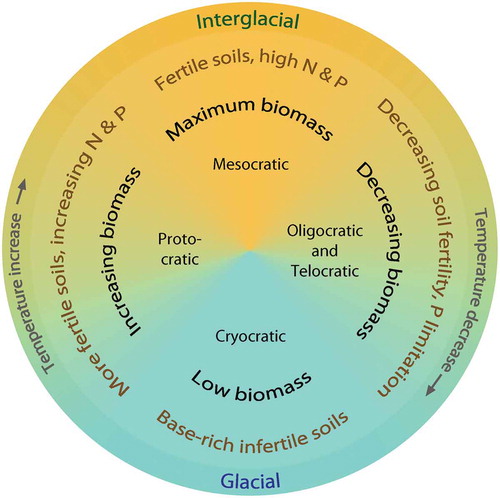
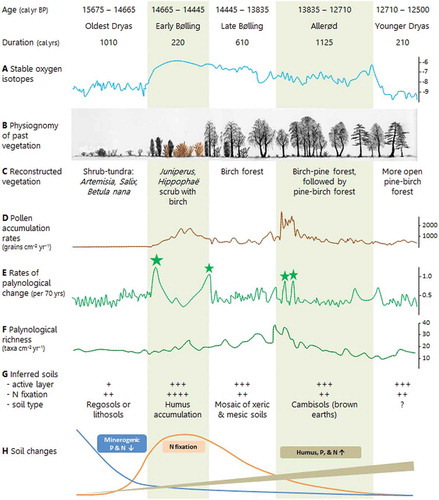
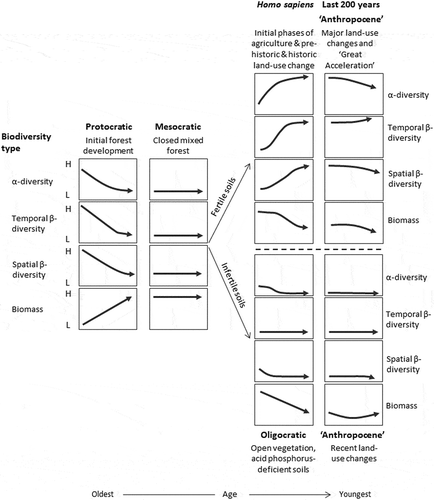
Table 3. Possible dominant mycorrhizal types and nitrogen fixers in a north-west European glacial–interglacial cycle and suggested levels of above-ground productivity, available soil phosphorus levels, and amount of nitrogen fixation. This scheme is based on many sources including Harley and Harley (Citation1987), Read (Citation1993a, Citation1993b), Cázares et al. (Citation2005), Kuneš et al. (Citation2011), and Dickie et al. (Citation2013).
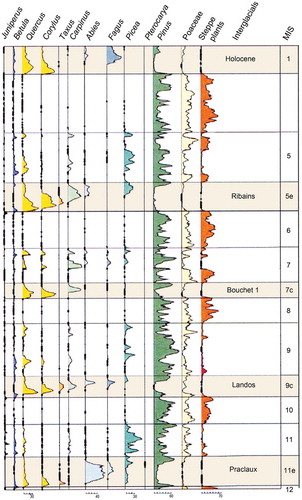
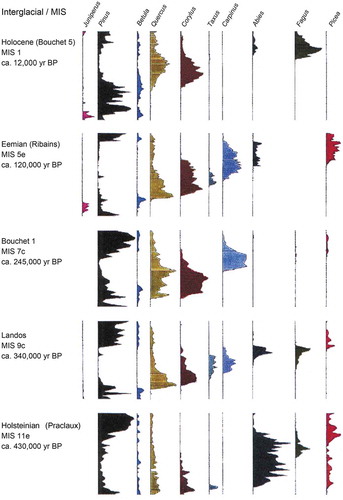
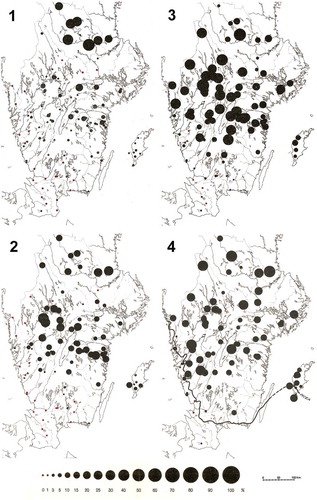
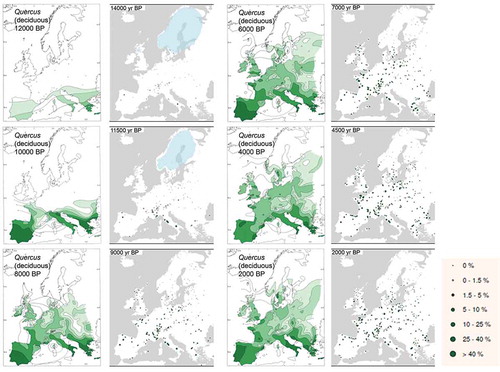
Table 4. Selected examples of Holocene isochrone maps for different geographical areas.
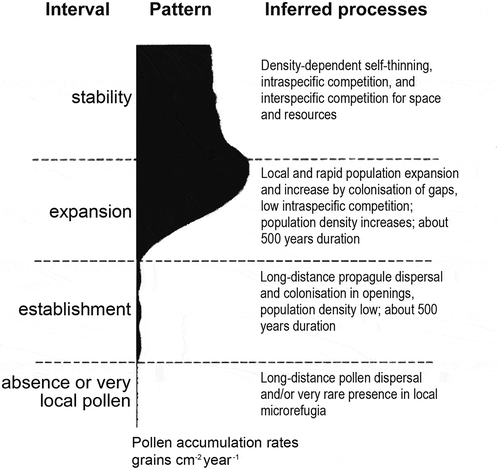
Table 6. Selected examples of range expansions or contractions of taxa that appear to show a ‘stop’ or ‘go’ behaviour during the Holocene.
Table 8. Comparison of modern climatic requirements of cool-temperate tree genera that grew in Europe in the Neogene but had been exterminated by the early Pliocene, that were present in Europe in the Neogene but have relictual disjunct distributions today, and that were present in the Neogene and are widespread in Europe today (based on Svenning Citation2003). Minimum growing degree days and the moisture index (actual evapotranspiration/potential evapotranspiration) are only available for genera in North America.
Table 10. Selected examples of publications on the role of persistence, evolutionary adaptation, and of phenotypic plasticity in influencing biodiversity patterns today and in the future.
Table 11. The frequency of the four modes of response in species populations and species combinations (‘associations’) within Europe and North America during the late-Quaternary.
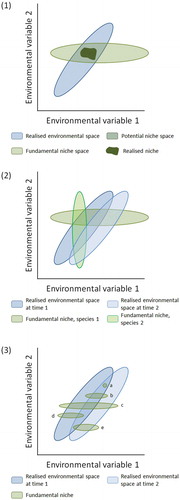
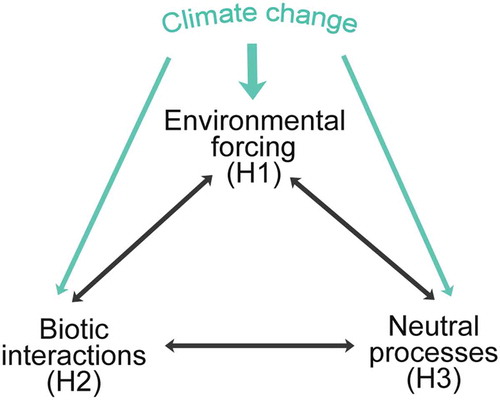
Table 12. Suggested drivers within the realised environment space (RES) resulting in no-analogue pollen assemblages and possible no-analogue vegetation types during different stages in the late-Quaternary in north-west Europe and eastern North America.
Table 13. Estimates of tree-population doubling times (td = logn(2/r); rounded to nearest 5 years) derived from pollen-stratigraphical data (PAR) from North America (Tsukada and Sugita Citation1982; Tsukada Citation1982c, Citation1982d; Bennett Citation1986, Citation1988a; MacDonald and Cwynar Citation1991; MacDonald Citation1993a; Fuller Citation1998; Edwards ME et al. Citation2015), England (Bennett Citation1983, Citation1988b), Central Europe (Giesecke et al. Citation2007; Bradshaw RHW et al. Citation2010), Italy (Magri Citation1989), north-east Australia (Walker and Chen Citation1987; Chen Citation1988), and Japan (Tsukada Citation1981, Citation1982a, Citation1982b, Citation1982c; Tsukada and Sugita Citation1982; Kito and Takimoto Citation1999). Modified from MacDonald (Citation1993a).
Table 14. Estimates of tree-population halving times (th = loge(2/r); rounded to nearest 5 years) derived from pollen-stratigraphical data (PAR) from North America (Tsukada and Sugita Citation1982), England (Peglar Citation1993a; Peglar and Birks Citation1993), and Japan (Tsukada Citation1983).
Table 15. Estimates of tree-population doubling times (td = loge(2/r); rounded to nearest 5 years) derived from pollen-stratigraphical data (PAR) from two sites in southern Ontario after the marked decline of Tsuga canadensis pollen at about 5400 years ago (calculated from data in Fuller Citation1998).
Table 16. Estimates of tree-population doubling times (td = loge(2/r); rounded to nearest 5 years) calculated from the eigenvalue (λ) of the transition matrix where logeλ = r (see Piñero et al. Citation1984). Data from various sources compiled and documented by Bennett (Citation1986).
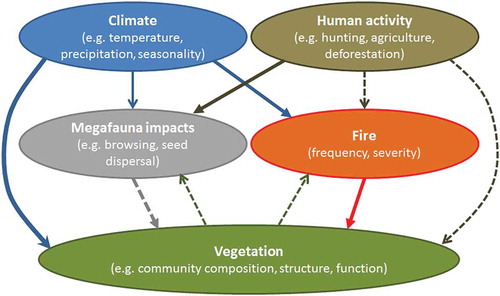
Table 17. Selected examples of detailed fire histories reconstructed from charcoal in Quaternary botanical sequences with emphasis on interactions of fire with other variables, and rapid state shifts and system dynamics.
Table 18. Selected examples of recent theoretical and Quaternary studies on ecosystem dynamics, in particular regime shifts.
Table 19. Approximate age of modern British woodland assemblages inferred from pollen-analytical stratigraphies and macrofossil data. The assemblages are based on the dominant tree taxa as indicated from pollen and/or plant macrofossil taxa. To aid vegetation reconstruction, fossil tree-pollen values have been transformed by Andersen’s (Citation1970) general pollen representation factors as modified for British taxa (Edwards ME Citation1986). The antecedent pollen assemblage or vegetation type are based on available dated pollen data from Britain (Birks HJB Citation1989; Bennett and Birks Citation1990; Fyfe et al. Citation2010, Citation2013; Brewer et al. Citation2017). For details of the NVC types, see Rodwell et al. (Citation1991).
Table 20. Selected examples of prehistoric human activities in the Amazon basin, lowland Congo basin, and Indo-Malaya region detected by palynological and associated palaeoecological studies with the approximate age of the activities and relevant references.
Table 21. Selected examples of publications on the potential contribution of Quaternary or ‘Deep-time’ palaeobiological studies to conservation by providing a historical perspective.
Table 22. Further examples of Quaternary palaeobiological studies that link directly to conservation and management that are not discussed in the text.
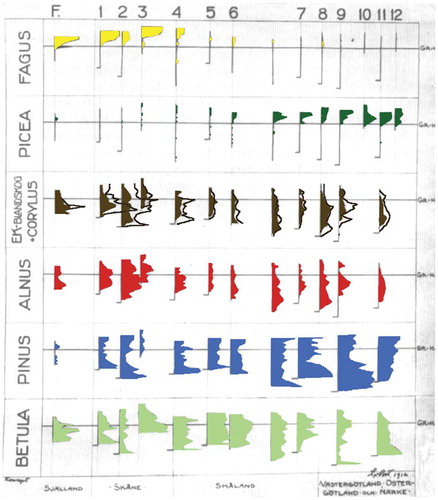
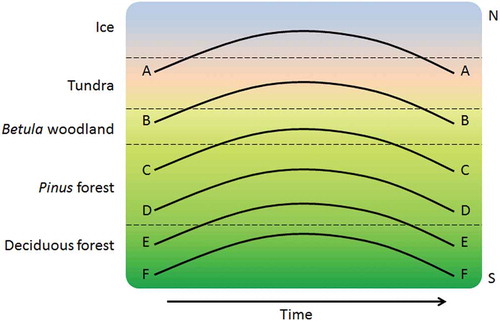
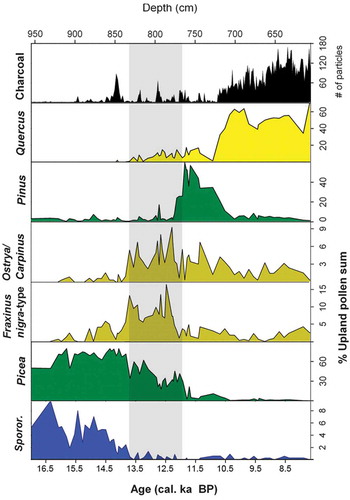
Table 23. Selected examples of detailed local- or regional-scale palynological studies on the history of specific vegetation types in Europe, Asia, and United States.
Table 24. Selected examples of palaeoecological studies that contribute to establishing baseline or reference conditions at a particular site or vegetation type.
Table 25. Comparison of ‘proximate’ and ‘ultimate’ diversity and stability on oceanic islands and continental mainlands (modified from Cronk Citation1997).
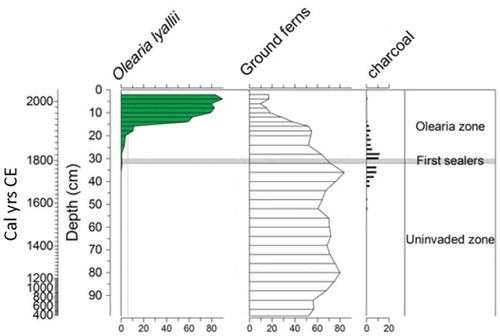
Table 26. Selected examples of islands or island groups in different oceans where pollen-analytical studies show compositional stability prior to the onset of human impact and ecosystem modification. The approximate duration of the periods with stable pollen composition is given, along with the age for the onset of human activities and an indication of plant extinctions or exterminations of all types on each island. The duration of the stable phases are minimal as the pollen assemblages in some of the sequences may commence within the stable phase.
Table 27. Recent contributions to the debate about the causes of Late Pleistocene and early-Holocene megafaunal extinctions.