Figures & data
Table 1. Clinical urine samples without known pneumococcal disease used for clinical specificity evaluation.
Table 2. IPD samples used for clinical sensitivity evaluation with serotyping results determined by blood culture isolates.
Figure 1. Development of the 21-valent SSUAD. (A) Schematic representation of the assay workflow, not drawn to scale. (B) Representative standard curves from four plates from selectivity testing. Vertical lines indicate the assay range.
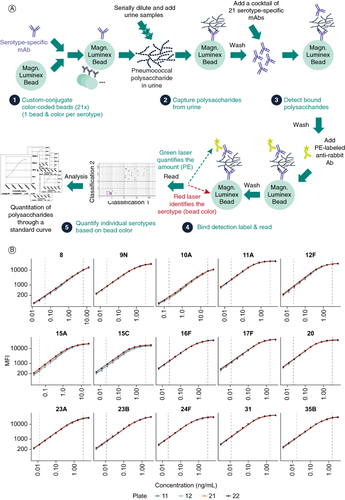
Table 3. Assay qualification performance in 21-valent assay.
Table 4. Consolidated table for parameters from serotype-specific urine antigen detection assay 21-valent assay qualification.
Table 5. Clinical positivity cut-offs and assay LOQs for 30 serotypes.
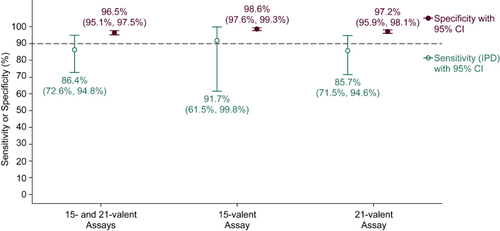
Figure 2. Clinical specificity and sensitivity to detect serotype-specific pneumococcal infection based on the presence of pneumococcal polysaccharides in patient urines. Clinical specificity and sensitivity were assessed for each assay individually and combined as indicated.