Figures & data
Figure 1. Root cause analysis for Case Study 1. (A) Spread of instrument responses from individual plasma samples from healthy and diseased individuals from cut point factor (CPF) calculations. Enhanced scatter of signals from diseased matrices caused a high CPF of 3.58. (B) Positive correlation between target A concentrations and signals in the ADA assay suggesting interference in the ADA assay by target A.
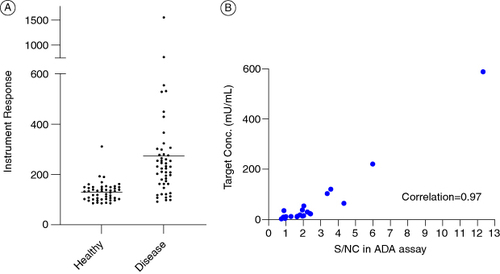
Figure 2. Testing masking reagents as mitigation for multimeric target interference. (A) Effect of various anti-target A masking reagents on the S/N ratio of a subset of individual human plasma lots used for the determination of the CPF. No significant impact on positive controls shown on top right. (B) Performance of the ADA assay with and without target masking antibody addition. Samples in blue on the left represent assay performed without any target A mitigation. The middle panel shows the same samples as the left but with the target A mitigation in place. The right-most graph shows the signals from all diseased individual samples tested during method development using the target A mitigation.
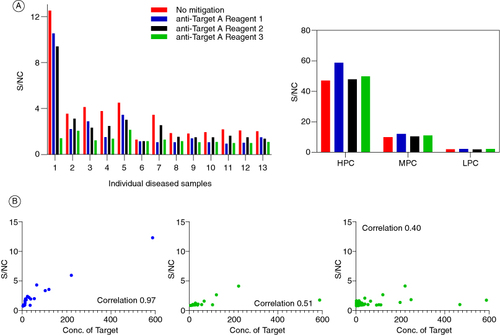
Table 1. Comparison of validation results from the original assay and the target A tolerant assay for Case study 1.
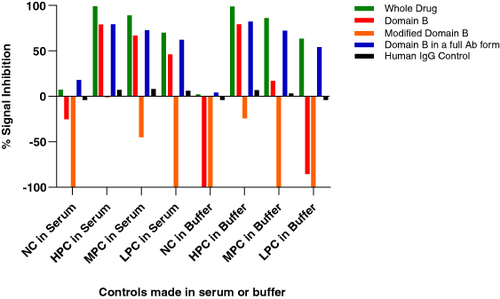
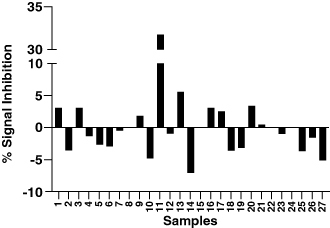
Figure 3. Signal inhibition percentages for whole drug and domain-specific confirmatory assays obtained from a set of 32 diseased human subjects. Addition of the drug or domain A to the confirmatory assay causes a signal decrease relative to the screening assay raw signals. On the contrary, addition of domain B causes a signal increase.
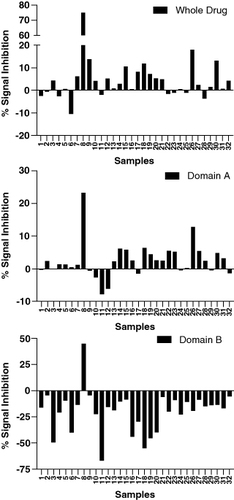
Figure 4. Different reagents used for troubleshooting the confirmatory assay. The standard confirmatory reagents shown in the top panel from left to right include the drug (full-length antibody containing domains A and B), domain A (fragment of the drug binding to target X), and domain B (fragment of the drug binding to target Y). The additional confirmatory reagents being utilized include a full-length antibody (bottom left) with domain B (same as the drug) and domain C (binding to an irrelevant target D); and a modified domain B (bottom right) where the paratope has been modified to bind to an irrelevant target Z. The polyclonal positive control (PC) has been purified to remove anti-drug antibodies against the humanized IgG backbone, resulting in the PC containing antibodies specifically targeting domains A and B. When the domain A+B (drug) is added, it binds to all the PCs, confirming the presence of antibodies against all domains of the drug. In contrast, the domain B+C molecule would only bind to the antibodies raised against domain B, with no signal reduction from the antibodies against domain A. The polyclonal PC mix does not contain antibodies against domain C.
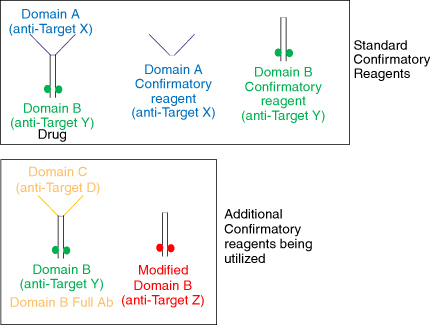
Figure 5. Performance of different domain B containing reagents in the anti-drug antibody confirmatory assay. Both the fragments cause signal increase compared with the screening assay, whereas the full-length antibodies show a signal decrease. Additionally, the signal increase caused by the fragments is higher in the absence of serum (positive controls made in buffer).