Figures & data
Table 1. Reimbursed biosimilars in Belgium, classified by treatment setting for use in non-hospitalised patients (as of January 2020) [Citation3]. Marketing authorization dates for the European Union are indicated between brackets [Citation2]. Molecules are sorted from oldest (on top) to newest biosimilar marketing authorization for the European Union. Please note that marketing authorization is not equal to access to treatment, since reimbursement and product launch usually occur later
Figure 1. Biosimilar market shares in Belgium, based on volume (defined daily doses) versus the reference product, for the years 2015 to 2019. Data for 2019 are partly extrapolated (EP) based on 6 months of hospital data and 9 months of data for the retail setting. Data were provided by the Belgian reimbursement agency RIZIV/INAMI/NIHDI (Farmanet and DOC PH databases)
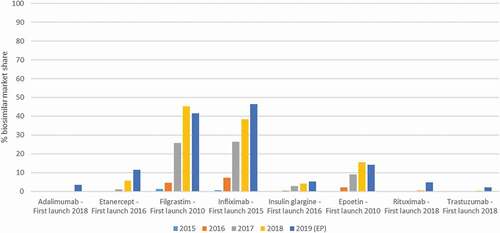
Table 2. Implemented policy measures related to biosimilars to enhance competition in the Belgian off-patent biologicals market
Table 3. Price differences between biosimilar and reference product in the retail setting (as of January 2020) [Citation36]
