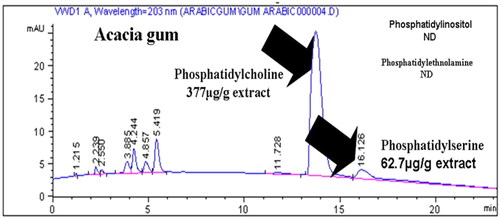
Figures & data
Table 1. Composition and calculated analyses of the basal diet (g/kg, as-fed basis) of weaned rabbits (Atallah et al. Citation2021).
Table 2. Average values of air temperature (T), relative humidity (RH), and temperature humidity index (THI) at the experimental site.
Table 3. Nutritional impacts of acacia gum, vitamin C, and lycopene on semen quality New Zealand rabbits under heat stress.
Figure 2. Nutritional impacts of acacia gum, vitamin C and lycopene on brain neurotransmitter New Zealand rabbits under heat stress. a–dMean values within the same box with different superscript letters are significantly different. p < 0.001.
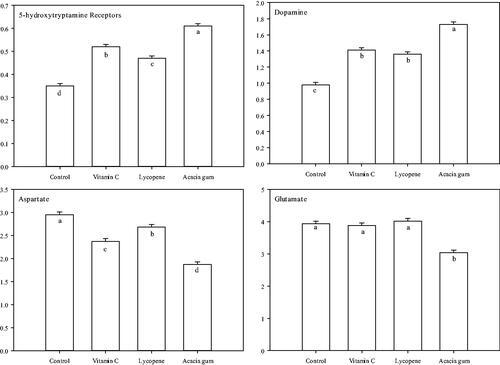
Table 4. Nutritional impacts of acacia gum, vitamin C, and lycopene on semen antioxidants New Zealand rabbits under heat stress.
Figure 3. The aromatic interaction and the 2D chemical interactions of the docked complexes between catalase enzyme and (A,B) lycopene, (C,D) vitamin-C, (E,F) phosphatidylserine, and (G,H) phosphatidylcholine.
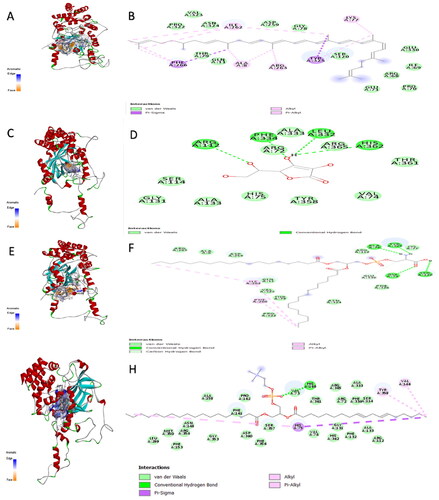
Figure 4. The aromatic interaction and the 2D chemical interactions of the docked complexes between glutathione peroxidase enzyme and (A,B) lycopene, (C,D) vitamin-C, (E,F) phosphatidylserine, and (G,H) phosphatidylcholine.
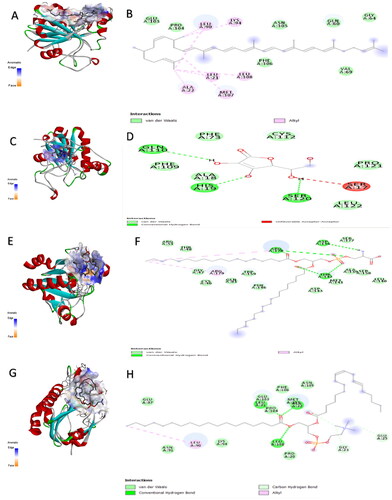
Figure 5. The aromatic interaction and the 2D chemical interactions of the docked complexes between superoxide dismutase enzyme and (A,B) lycopene, (C,D) vitamin-C, (E,F) phosphatidylserine, and (G,H) phosphatidylcholine.
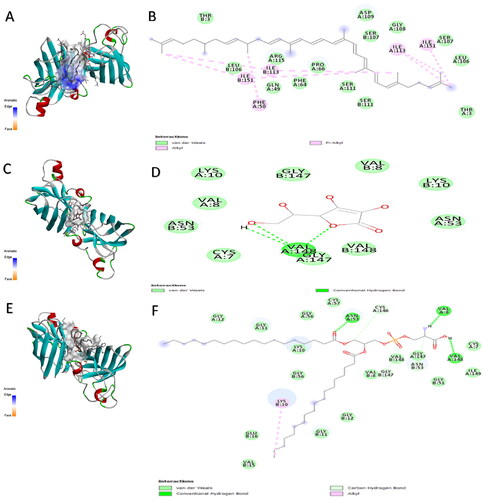
Figure 6. The H-bond acceptor in green and doner in pink colours, charges positive with blue and negative with red colours, a hydrophobic area with a brown and hydrophilic area with blue colours, ionizability with basic in blue and acidic in red colours, and solvent accessible surface (SAS) in blue colour through the interactions of the docked complexes between catalase enzyme and (A–E) lycopene, (F–J) vitamin-C, (K–O) phosphatidylserine, and (P–T) phosphatidylcholine, respectively.
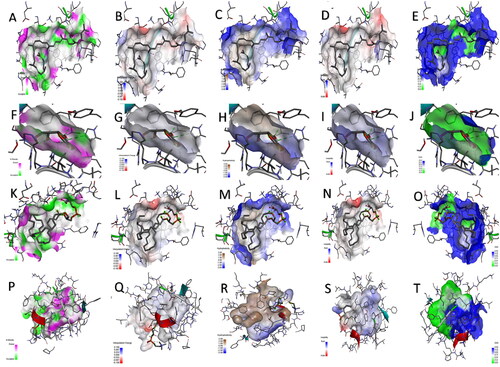
Figure 7. The H-bond acceptor in green and doner in pink colours, charges positive with blue and negative with red colours, a hydrophobic area with a brown and hydrophilic area with blue colours, ionizability with basic in blue and acidic in red colours, and solvent accessible surface (SAS) in blue colour through the interactions of the docked complexes between glutathione peroxidase enzyme and (A–E) lycopene, (F–J) vitamin-C, (K–O) phosphatidylserine, and (P–T) phosphatidylcholine, respectively.
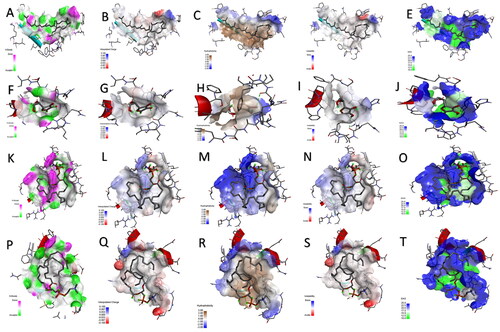
Figure 8. The H-bond acceptor in green and doner in pink colours, charges positive with blue and negative with red colours, a hydrophobic area with a brown and hydrophilic area with blue colours, ionizability with basic in blue and acidic in red colours, and solvent accessible surface (SAS) in blue colour through the interactions of the docked complexes between superoxide dismutase enzyme and (A–E) lycopene, (F–J) vitamin-C, (K–O) phosphatidylserine, and (P–T) phosphatidylcholine, respectively.
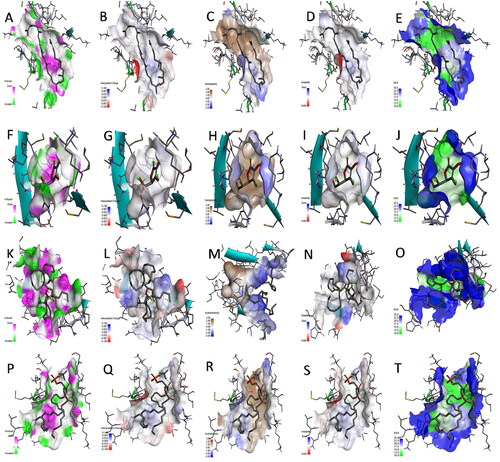
Table 5. The docking analysis results of binding energy, No. of H-bond, No. of carbon H-bond, hydrophobicity, and SAS interactions between GSH, SOD, and CAT enzymes against vitamin C, lycopene, phosphatidylcholine, and phosphatidylserine.