Figures & data
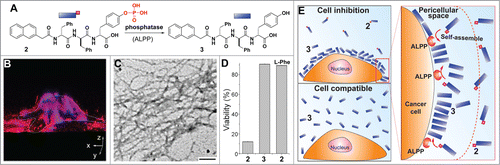
Figure 1. PriSM of 1 selectively inhibit cancer cells. (A) The structure of a building block (1) of PriSM. (B) ThT binding assays of as-prepared solution of 1. The ThT emission starts to increase with the concentration of 1 in a linear manner above the concentration of 163 μg/mL. The inset is the 48 h viability (MTT assay) of HeLa cells treated with as-prepared 1. (C) MHPB assay: SDS-PAGE; major proteins from MS protein profiling; Western blot confirms tubulins as the primary targets of PriSM of 1, and PriSM of 1 interacting with multiple proteins (lanes: complete cell lysate (C), wash-off proteins (W3), proteins bound on the molecular nanofibers (MN)). (D) Confocal images of tubulin staining of HeLa cells treated with PriSM of 1 to confirm PriSM of 1 disrupting the elongation process of microtubules (scale bar = 10 µm). (E) PriSMs of 1 enter the cell by macropinocytosis, impede the cytoskeletal proteins, and result in apoptosis.
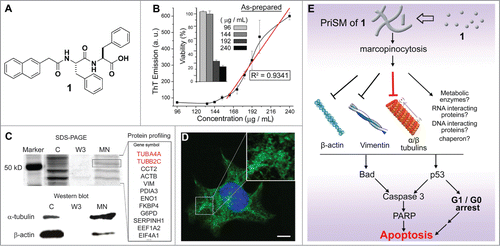
Figure 2. PriSM formed in pericellular space for selectively inhibiting cancer cells. (A) The structures of a precursor (2) and the self-assembling small molecule (3). (B) 3D stacked z-scan fluorescent images of Congo red stained PriSM on HeLa cells incubated with 2 (400 μg/mL) (C) TEM images of the PriSM of 3 on the cells (scale bar = 100 nm). (D) Cell viabilities of HeLa treated by 2 or 3 at 200 µg/mL, or 2 (217 µg/mL)+L-Phe (54 µg/mL) for 48h. (E) ALPP-instructed formation of PriSM of 3 on cell surface inhibits the cancer cells, but 3, as the soluble monomers, is innocuous to cells. Adapted from Refs. Citation16 and Citation19. © 2014 John Wiley & Sons Inc. Reproduced by permission of John Wiley & Sons Inc. Permission to reuse must be obtained from the rightsholder.