Figures & data
TABLE 1. Species susceptibility to CWD infection following experimental exposure via the intracerebral (IC) or oral (PO) routes of exposure.
FIGURE 1. Investigating the structural determinants of the CWD-human transmission barrier. The human and elk β2-α2 loop amino acid sequences differ at 4 positions: 166, 168, 170, and 174 (top). Transgenic mice expressing full-length human PrPC (blue) or human PrPC with the elk β2-α2 loop (red) were inoculated intracerebrally with CWD prions. Although mice expressing human PrPC did not develop disease, mice expressing the human-elk loop PrPC [Tg(HuPrPelk166-174)] were susceptible to CWD infection (83%). Inoculation of brain from a CWD-infected Tg(HuPrPelk166-174) mouse into additional transgenic mice transmitted the disease to all Tg(HuPrPelk166-174) mice, but to only 1 of 17 Tg(HuPrP) mice.
![FIGURE 1. Investigating the structural determinants of the CWD-human transmission barrier. The human and elk β2-α2 loop amino acid sequences differ at 4 positions: 166, 168, 170, and 174 (top). Transgenic mice expressing full-length human PrPC (blue) or human PrPC with the elk β2-α2 loop (red) were inoculated intracerebrally with CWD prions. Although mice expressing human PrPC did not develop disease, mice expressing the human-elk loop PrPC [Tg(HuPrPelk166-174)] were susceptible to CWD infection (83%). Inoculation of brain from a CWD-infected Tg(HuPrPelk166-174) mouse into additional transgenic mice transmitted the disease to all Tg(HuPrPelk166-174) mice, but to only 1 of 17 Tg(HuPrP) mice.](/cms/asset/3180df98-b9f3-41a6-af4b-d16c74af5792/kprn_a_1118603_f0001_c.gif)
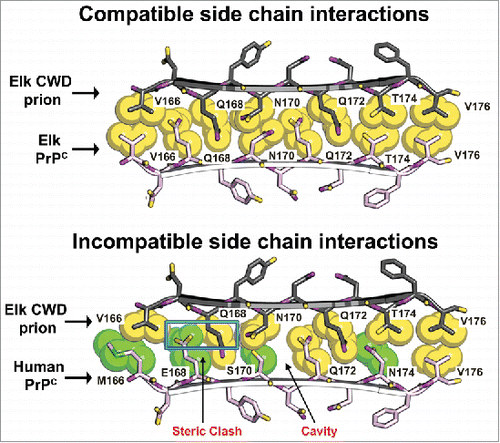
FIGURE 2. Structural models of elk and human side chain packing within the β2-α2 loop may explain CWD transmission barriers. Atomic space-filling models of amino acid side chains within the β2-α2 loop of PrP were modeled as a parallel β-sheet.Citation53 In this model, the CWD PrPSc and cervid PrPC (top pair) interdigitate in a steric zipper. In contrast, the CWD PrPSc and human PrPC (bottom pair) interaction generates a steric clash (blue rectangle) and a cavity (arrow) that would be incompatible with zipper formation and may explain why CWD does not convert human PrPC. Amino acids common to both cervids and humans are yellow; human-specific residues are green.