Figures & data
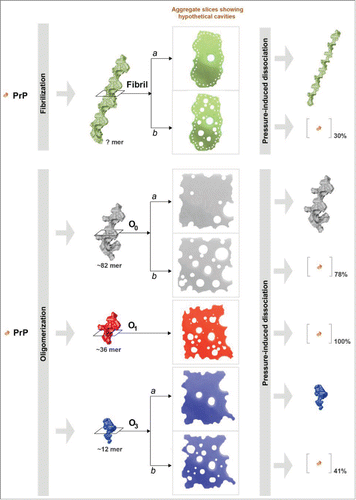
Figure 1. Solubilized brain material from uninfected (black) or 263K (red) infected hamsters was fractionated by sedimentation at the equilibrium. The collected fractions (numbered from top to bottom of the gradient) were analyzed for PrP content by immunoblot. The amount (in ng) of PrP per fraction was reported on the graph by using a standard curve of recombinant PrP. For methodological details see ref. Citation34.
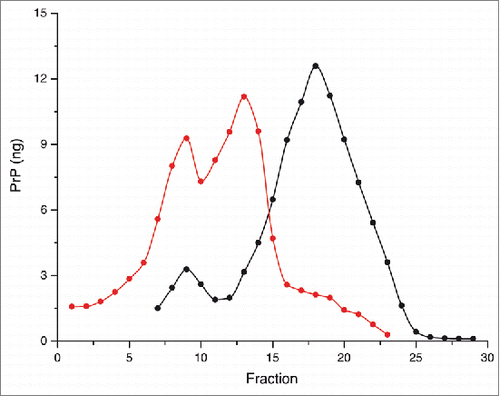
Figure 2. Schematic representation of the effects of high pressure on mature PrP amyloid fibrils and 3 distinct β-sheet-rich PrP oligomers. This diagram illustrates the proposed model of pressure-induced structural changes, which depends on the strongly varying cavities present in their quaternary structure. The amount (% on the entire protein sample) of resolubilized PrP rescued from the superstructure after the pressure treatment is also shown.