Figures & data
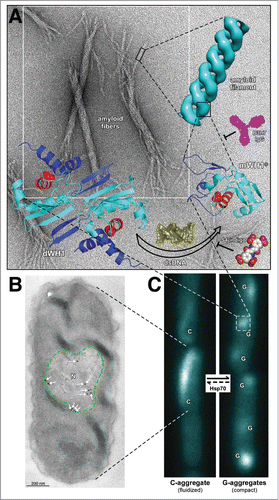
Figure 1 (See next page). Overview of RepA-WH1 amyloidogenesis, remarking hierarchical assembly in vitro (A) and phase transitions in vivo (B,C). (A) Stable dimers of RepA-WH1 (dWH1) undergo a structural transformation upon transient, low affinity binding to dsDNA, thus resulting in metastable, aggregation-prone monomers (mWH1*).Citation13 The core of the WH domain is colored cyan, whereas segments showing significant conformational changes are in blue. The amyloidogenic peptide L26VLCAVSLI34 is depicted in red, with the side-chain of the hyper-amyloidogenic mutant residue A31V shown as spheres.Citation17 Binding of dsDNA (yellow) to dWH1 disrupts the dimerization interface, thus generating partially unfolded mWH1* monomers which assemble as helical amyloid tubular filaments.Citation17,27 Binding of RepA-WH1 to dsDNA, and thus amyloidogenesis, can be competed by molecules of S4-indigo (spheres),Citation18 whereas the conformation specific antibody B3h7 (magenta) inhibits the assembly of RepA-WH1 oligomers into filaments.Citation38 Filaments further associate laterally and coil to generate the mature amyloid fibers (background EM).Citation17,27 (B) Electron micrograph showing an ultra-thin section through an E. coli cell incubated with the B3h7 antibody (arrows/dots: gold-conjugated secondary anti-mouse antibody), which reveals the preferential location of pre-amyloidogenic RepA-WH1 aggregates at the nucleoid (N; green dashed line).Citation38 A fluidized C-type aggregate hydrogel is also outlined (cyan dotted line).Citation39 (C) E. coli cells growing in microfluidic channels and expressing the prionoid.Citation39 RepA-WH1 amyloid aggregates show two distinct appearances, i.e. single comet-like (C) aggregates behaving as a fluidized hydrogel that readily splits on cell division; or multiple globular (G) cytotoxic aggregates with the compactness characteristic of typical amyloid plaques.Citation39 While the phase transition from the C to the G aggregates occurs spontaneously in vivo, the reverse uphill transition is promoted by a single cell factor: the Hsp70 chaperone DnaK.Citation39